Atelocollagen Honeycomb sponge
KOKEN
- Catalog No.:
- KOU-CSH-10
- Shipping:
- Calculated at Checkout
$680.00
US Customers: This item requires a permit. Click here for more information.
Background
Honeycomb Sponge is made from type I atelocollagen and possess honeycomb-like pore structure with high pore density. This honeycomb structure enables easy supply of nutrients to cells and excretion of waste products from cells.
Applications
- 3D cell culture
- Scaffold for regenerative medicine research
- Sustained release of bioactive substances
Features
- Unidirectional pore structure facilitates cell migration and vascularization in vivo
- Cells can be easily harvested by collagenase treatment.
This product is manufactured in Japan and produced from raw materials obtained from livestock certified born and raised in Australia or New Zealand. Japan, Australia and New Zealand are all recognized by the World Health Organization as having negligible risk for Bovine Spongiform Encephalitis (BSE). Nevertheless, please consult with your country's Customs office to confirm importability.
| Documents & Links for Atelocollagen Honeycomb sponge | |
| Datasheet | Atelocollagen Honeycomb sponge Datasheet |
| Flyer | Atelocollagen Honeycomb sponge Flyer |
| Documents & Links for Atelocollagen Honeycomb sponge | |
| Datasheet | Atelocollagen Honeycomb sponge Datasheet |
| Flyer | Atelocollagen Honeycomb sponge Flyer |







![Implantation of bone marrow stem cells (BMSCs) into hemisected spinal cord using CSH (Enomoto M, Department of Orthopaedic Surgery and Hyperbaric Medical Center, Tokyo Medical and Dental University)
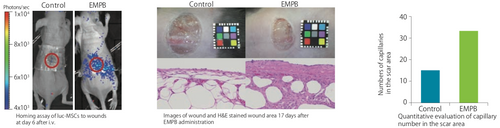
A previous report demonstrated that the pore structure of CSH enhances nerve regeneration (Ref. 9). In this research, a remarkable increase of neurite growth was observed when dorsal root ganglia (DRG)s were cultured on GEP-expressing BMSCs contained CSH (BMSCs+CSH) for ten days compared to DRGs cultured on CSH alone. Improved locomotor and sensory function was also observed four weeks after implantation of BMSC+CSH into hemisected spinal cord (Ref. 4). [Tuj-1: A maker for neuron; SMI31: A marker for neurofilament; GFAP: a marker for astrocyte; BBB score: an evaluation method for hindlimb motor function] Implantation of bone marrow stem cells (BMSCs) into hemisected spinal cord using CSH (Enomoto M, Department of Orthopaedic Surgery and Hyperbaric Medical Center, Tokyo Medical and Dental University)
A previous report demonstrated that the pore structure of CSH enhances nerve regeneration (Ref. 9). In this research, a remarkable increase of neurite growth was observed when dorsal root ganglia (DRG)s were cultured on GEP-expressing BMSCs contained CSH (BMSCs+CSH) for ten days compared to DRGs cultured on CSH alone. Improved locomotor and sensory function was also observed four weeks after implantation of BMSC+CSH into hemisected spinal cord (Ref. 4). [Tuj-1: A maker for neuron; SMI31: A marker for neurofilament; GFAP: a marker for astrocyte; BBB score: an evaluation method for hindlimb motor function]](https://cdn11.bigcommerce.com/s-ydswqc5qsc/images/stencil/500x659/products/40927/184023/kou-csh-10_atelocollagen-honeycomb-sponge_7954__20623.1708393899.png?c=2)
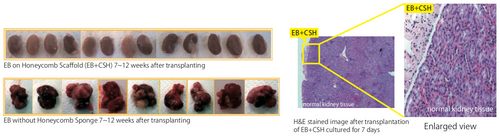
![Induction of hepatic differentiation of ES cells and transplantation using CSH
Embryoid bodies (EBs) were formed from ES cells and then inserted into CSH. EBs both with and without CSH were cultured to differentiate and induce hepatic histogenesis. The EB-derived cells expressed liver-specific genes, and albumin-positive cells formed cordlike structures that were not present in those without CSH. The scaffold including EB-derived hepatocyte-like cells was transplanted into the median lobe of mice. After 14 days, cells positive for both albumin and cytokeratin 18 appeared in the transplant and formed clustered aggregates. (Ref. 10) [AFP: Marker for inicial livers hepatic cells; ALB: Marker for inicial to matured hepatic cells; G6P and CK18: Marker for matured hepatic cells] Induction of hepatic differentiation of ES cells and transplantation using CSH
Embryoid bodies (EBs) were formed from ES cells and then inserted into CSH. EBs both with and without CSH were cultured to differentiate and induce hepatic histogenesis. The EB-derived cells expressed liver-specific genes, and albumin-positive cells formed cordlike structures that were not present in those without CSH. The scaffold including EB-derived hepatocyte-like cells was transplanted into the median lobe of mice. After 14 days, cells positive for both albumin and cytokeratin 18 appeared in the transplant and formed clustered aggregates. (Ref. 10) [AFP: Marker for inicial livers hepatic cells; ALB: Marker for inicial to matured hepatic cells; G6P and CK18: Marker for matured hepatic cells]](https://cdn11.bigcommerce.com/s-ydswqc5qsc/images/stencil/500x659/products/40927/184025/kou-csh-10_atelocollagen-honeycomb-sponge_7955__64851.1708393908.png?c=2)
