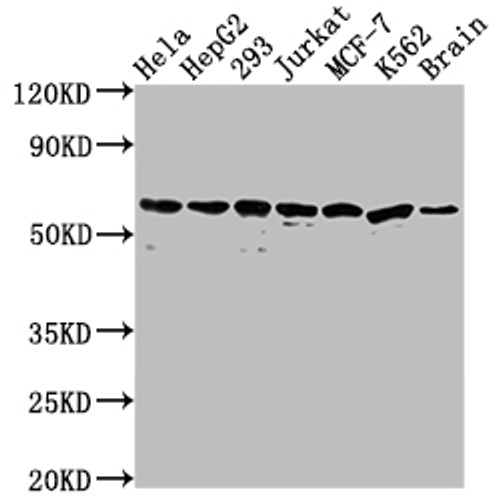
Anti HSPD1 mAb (Clone 3D8)
CUSABIO
- Catalog No.:
- CSB-RA953395A0HU-100
- Shipping:
- Calculated at Checkout
$368.00
| Product Specifications | |
| Application | WB, IHC, IP, ELISA |
| Reactivity | Human, Mouse |
| Clonality | Monoclonal (Clone No.: 3D8) |
| Documents & Links for Anti HSPD1 mAb (Clone 3D8) | |
| Datasheet | Anti HSPD1 mAb (Clone 3D8) Datasheet |
| Documents & Links for Anti HSPD1 mAb (Clone 3D8) | |
| Datasheet | Anti HSPD1 mAb (Clone 3D8) Datasheet |