SELECTION BY Product / Atelocollagen Neutral Solutions

Atelocollagen Neutral Solutions
Atelocollagen neutral solutions are high purity collagen solutions prepared from bovine dermis and form a gel under physiological conditions. These products are pre-mixed with culture medium and ready to use for cell culture.Application and Features
 |
| Culture on collagen gel |
- Browse more than 60 products related to ubiquitin Proteasome Research
- Featured products include the widely utilized monoclonal antibody clone FK1 (not reactive with methyl-ubiquitinated protein or free-ubiquitin, and clone FK2 (reactive with methyl ubiquitinated protein and not reactive with free ubiquitin).
- Atelocollagen neutral solutions are adjusted to neutral pH and form a gel when they are warmed to 37°C.
| Product name (click for order info) | Cat no (click for datasheet) | Size | |
| Atelocollagen, DMEM Low Glucose | KOU-DME-02 | 20 ml | |
| Atelocollagen, DMEM High Glucose | KOU-DME-02H | 20 ml | |
| Atelocollagen, RPMI 1640 | KOU-RPM-02 | 20 ml | |
Frequently Asked Questions
What are the differences between I-PC and I-AC?
I-PC is protease solubilised atelocollagen whose immunogenicity is reduced while maintaining the functions of collagen. I-PC is suitable both for gel formation and for collagen coating because of its relatively low viscosity. On the other hand, I-AC is acid-soluble collagen which retains telopeptides. Its viscosity, transparency and rigidity are higher than those of I-PC.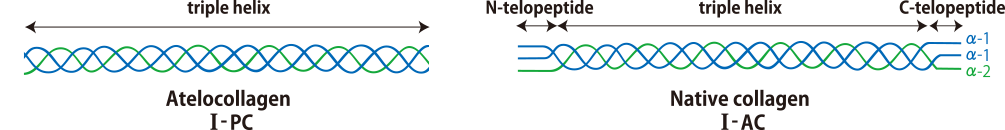
What is atelocollagen?
Telopeptide is a non-helical structure at both ends of the collagen molecule and is thought to be involved in intermolecular bonding and allergenic reaction due to antigenicity. Removing the telopeptides from collagen by protease treatment produces atelocollagen whose characteristics are superior purity and stability. We have produced atelocollagen-based medical devices for more than 30 years.
What types of collagen are contained in Collagen acidic solutions and Collagen neutral solutions?
The source of collagen is bovine dermis and therefore both products contain approximately 95% type I collagen and roughly 5% type III and type V collagen.
What is the difference between collagen acidic solutions and collagen neutral solutions?
Collagen is highly stable in acidic conditions and is appropriate for long-term storage. In contrast, collagen forms a gel under physiological conditions and thus must be neutralized and mixed with medium. This is why we have prepared Collagen neutral solution and 3D ready atelocollagen which readily form a gel upon warming. The collagen contained in Collagen neutral solution and 3D ready atelocollagen are atelocollagen.
How can I prepare collagen coatings/collagen gels?
Please visit our distributors’ page to download an instruction manual
How can I prevent cells from sinking during collagen gel formation?
When a low concentration of collagen is used, the viscosity is low and the gelling time is longer. We recommend using 5 mg/mL Atelocollagen or Native collagen acidic solution (Cat No. IPC-50 or IAC-50) or 3D Ready Atelocollagen (Cat No. 3D-LG01 or 3D-HG01).
How can I harvest cells from collagen gel?
Add collagenase to final concentration of 0.1% and incubate at 37°C for approximately an hour.
Is it possible to prepare sections from collagen gel?
Similar to tissue samples, collagen gel can be fixed and/or embedded in paraffin/OCT compound.
Is there a high concentration collagen solution that is ideal for cell transplantation?
We can provide a collagen solution equivalent to that used in manufacturing our atelocollagen-based medical devices as a made-to-order research product. Alternatively, a high concentration collagen solution can be prepared from Atelocollagen powder (Cat No. CLP-01).
What are the differences between soluble basement membrane extracts and collagen solution?
Our collagen solution does not contain cell-derived physiologically active compounds, nucleic acids, matrix metalloproteinase (MMP), etc., which could affect the results of your experiment. Unlike soluble basement membrane extracts, we confirm the lot-to-lot consistency of our collagen products.
References
3D culture
- An in vitro three-dimensional co-culture system for ameloblastoma modelling
Soo Leng Lee, Zainal Ariff Abdul Rahman, Hidetsugu Tsujigiwa, Mei Hamada, Kiyofumi Takabatake, Keisuke Nakano, Hitoshi Nagatsuka, Chong Huat Siar
Sains Malaysiana 48(8)(2019): 1697–1706. - Enhancement of HGF-induced tubulogenesis by endothelial cell-derived GDNF.
Nakasatomi M, Takahashi S, Sakairi T, Ikeuchi H, Kaneko Y, Hiromura K, Nojima Y, Maeshima A
PLoS One. 2019 Mar 7;14(3):e0212991. PMID: 30845150 - Osteogenic Potential of Mouse Periosteum-Derived Cells Sorted for CD90 In Vitro and In Vivo.
Kim YK, Nakata H, Yamamoto M, Miyasaka M, Kasugai S, Kuroda S.
Stem Cells Transl Med. 2016 Feb;5(2):227-34. PMID: 26718647 - Investigation of VEGF and PDGF signals in vascular formation by 3D culture models using mouse ES cells.
Hitomi Hosoe, Yuri Yamamoto, Yusuke Tanaka, Mami Kobayashi, Nana Ninagawa, Shigeko Torihashi.
Stem Cell Discovery. 2012 2(2):70-77. - Age-related decline in label-retaining tubular cells: implication for reduced regenerative capacity after injury in the aging kidney.
Miya M, Maeshima A, Mishima K, Sakurai N, Ikeuchi H, Kuroiwa T, Hiromura K, Nojima Y.
Am J Physiol Renal Physiol. 2012 Mar 15;302(6):F694-702. PMID: 22169012 - Enhancement of in vitro human tubulogenesis by endothelial cell-derived factors: implications for in vivo tubular regeneration after injury.
Miya M, Maeshima A, Mishima K, Sakurai N, Ikeuchi H, Kuroiwa T, Hiromura K, Yokoo H, Nojima Y.
Am J Physiol Renal Physiol. 2011 Aug;301(2):F387-95. PMID: 21561997 - Activin A as a critical mediator of capillary formation: interaction with the fibroblast growth factor action.
Hayashi Y, Maeshima K, Goto F, Kojima I.
Endocr J. 2007 Apr;54(2):311-8. PMID:17384470 - Application of periodontal ligament cell sheet for periodontal regeneration: a pilot study in beagle dogs.
Akizuki T, Oda S, Komaki M, Tsuchioka H, Kawakatsu N, Kikuchi A, Yamato M, Okano T, Ishikawa I.
J Periodontal Res. 2005 Jun;40(3):245-51.PMID: 15853971 - Pkd1 regulates immortalized proliferation of renal tubular epithelial cells through p53 induction and JNK activation.
Nishio S,Hatano M, Nagata M, Horie S, Koike T, Tokuhisa T, Mochizuki T.
J Clin Invest. 2005 Apr;115(4):910-8. PMID: 15761494 - Involvement of renal progenitor tubular cells in epithelial-to-mesenchymal transition in fibrotic rat kidneys.
Yamashita S,Maeshima A, Nojima Y.
J Am Soc Nephrol. 2005 Jul;16(7):2044-51. PMID: 15888566 - Repair of articular cartilage defect by autologous transplantation of basic fibroblast growth factor gene-transduced chondrocyteswith adeno-associated virus vector.
Yokoo N, Saito T, Uesugi M, Kobayashi N, Xin KQ, Okuda K, Mizukami H, Ozawa K, Koshino T.
Arthritis Rheum. 2005 Jan;52(1):164-70. PMID: 15641065 - Crucial role of activin a in tubulogenesis of endothelial cells induced by vascular endothelial growth factor.
Maeshima K, Maeshima A, Hayashi Y, Kishi S, Kojima I.
Endocrinology. 2004 Aug;145(8):3739-45. PMID: 15117880 - JAM4 enhances hepatocyte growth factor-mediated branching and scattering of Madin-Darby canine kidney cells.
Mori H, Hirabayashi S,Shirasawa M, Sugimura H, Hata Y.
Genes Cells. 2004 Sep;9(9):811-9. PMID: 15330858 - JAM4, a junctional cell adhesion molecule interacting with a tight junction protein, MAGI-1.
Hirabayashi S, Tajima M, Yao I, Nishimura W,Mori H, Hata Y.
Mol Cell Biol. 2003 Jun;23(12):4267-82. PMID: 12773569 - Autocrine stimulation by osteopontin contributes to antiapoptotic signalling of melanocytes in dermal collagen.
Geissinger E, Weisser C, Fischer P, Schartl M, Wellbrock C.
Cancer Res. 2002 Aug 15;62(16):4820-8. PMID: 12183442 - Gene marking in adeno-associated virus vector infected periosteum derived cells for cartilage repair.
Kobayashi N, Koshino T, Uesugi M, Yokoo N, Xin KQ, Okuda K, Mizukami H, Ozawa K, Saito T.
J Rheumatol. 2002 Oct;29(10):2176-80.PMID: 12375329 - Role of HGF/c-met system in invasion and metastasis of oral squamous cell carcinoma cells in vitro and its clinical significance.
Uchida D,Kawamata H, Omotehara F, Nakashiro Ki, Kimura-Yanagawa T, Hino S, Begum NM, Hoque MO, Yoshida H, Sato M, Fujimori T.
Int J Cancer.2001 Aug 15;93(4):489-96. PMID: 11477552 - Role of CD44 variant exon 6 in invasion of head and neck squamous cell carcinoma.
Kanke M, Fujii M, Kameyama K, Kanzaki J, Tokumaru Y,Imanishi Y, Tomita T, Matsumura Y.
Arch Otolaryngol Head Neck Surg. 2000 Oct;126(10):1217-23.PMID: 11031408
Fabricating scaffold, miscellaneous
- Distinguishing heat-treated dead cells from viable cells using frequency dependence of electrical impedance.
Fujimaki M, Sei R, Yokoseki K, Nebuya S, Sakai R, Yoshida K, Ujihira M.
Biomed Mater Eng. 2022;33(4):315-324. PMID: 35180102. - Cell fiber-based 3D tissue array for drug response assay.
Kato-Negishi M, Sawayama J, Kawahara M, Takeuchi S.
Sci Rep. 2022 May 12;12(1):7870. PMID: 35552465. - Comparison of cultured cell attachment on a temperature-responsive polymer, poly-L-lysine, and collagen using modeling curves and a thermal-controlled quartz crystal microbalance.
Alsaleem AHA, Ito S, Naemura K, Muramatsu H
J Biol Phys. 2021 Jun;47(2):117-129. PMID: 33893599 - Long-Term Effect of Honeycomb β-Tricalcium Phosphate on Zygomatic Bone Regeneration in Rats.
Nakagiri R, Watanabe S, Takabatake K, Tsujigiwa H, Watanabe T, Matsumoto H, Kimata Y.
Materials (Basel). 2020 Nov 26;13(23):5374. PMID: 33256248. - Cell-laden microfibers fabricated using μl cell-suspension.
Nie M, Nagata S, Aoyagi H, Itou A, Shima A, Takeuchi S.
Biofabrication. 2020 Aug 5;12(4):045021. PMID: 32299072. - Microfluidic Device for the Analysis of Angiogenic Sprouting under Bidirectional Biochemical Gradients.
Nishimura K, Nie M, Miura S, Takeuchi S.
Micromachines (Basel). 2020 Nov 27;11(12):E1049. PMID: 33261134. - Effect of Honeycomb β-TCP Geometrical Structure on Bone Tissue Regeneration in
Watanabe T, Takabatake K, Tsujigiwa H, Watanabe S, Nakagiri R, Nakano K, Nagatsuka H, Kimata Y.
Skull Defect. Materials (Basel). 2020 Oct 25;13(21):4761. PMID: 33113818. - Rod-Shaped Neural Units for Aligned 3D Neural Network Connection.
Kato-Negishi M, Onoe H, Ito A, Takeuchi S.
Adv Healthc Mater. 2017 Aug;6(15). PMID: 28429415 - Parkin promotes proteasomal degradation of synaptotagmin IV by accelerating polyubiquitination.
Kabayama H, Tokushige N, Takeuchi M, Kabayama M, Fukuda M, Mikoshiba K.
Mol Cell Neurosci. 2017 Apr;80:89-99.PMID: 28254618 - ASF-4-1 fibroblast-rich culture increases chemoresistance and mTOR expression of pancreatic cancer BxPC-3 cells at the invasive front in vitro, and promotes tumor growth and invasion in vivo.
Fujiwara M, Kanayama K, Hirokawa YS, Shiraishi T.
Oncol Lett. 2016 Apr;11(4):2773-2779. PMID: 27073551 - Long-term gene therapy with Del1 fragment using nonviral vectors in mice with explanted tumors.
Kitano H, Mamiya A, Ishikawa T, Egoshi K, Kokubun S, Hidai C.
Onco Targets Ther. 2016 Jan 25;9:503-16. PMID: 26889088 - Differentiation Induction of Mouse Neural Stem Cells in Hydrogel Tubular Microenvironments with Controlled Tube Dimensions.
Onoe H, Kato-Negishi M, Itou A, Takeuchi S.
Adv Healthc Mater. 2016 May;5(9):1104-11. PMID: 26919482 - Smooth muscle-like tissue constructs with circumferentially oriented cells formed by the cell fiber technology.
Hsiao AY, Okitsu T, Onoe H, Kiyosawa M, Teramae H, Iwanaga S, Kazama T, Matsumoto T, Takeuchi S.
PLoS One. 2015 Mar 3;10(3):e0119010. PMID: 25734774 - Neural stem/progenitor cell-laden microfibers promote transplant survival in a mouse transected spinal cord injury model.
Sugai K, Nishimura S, Kato-Negishi M, Onoe H, Iwanaga S, Toyama Y, Matsumoto M, Takeuchi S, Okano H, Nakamura M.
J Neurosci Res. 2015 Dec;93(12):1826-38. PMID: 26301451 - Hepatocyte Selection Medium.
Minoru Tomizawa, Fuminobu Shinozaki, Yasufumi Motoyoshi, Takao Sugiyama, Shigenori Yamamoto and Makoto Sueishi.
Pluripotent Stem Cell Biology - Advances in Mechanisms, Methods and Models. Prof. Craig Atwood (Ed.), ISBN: 978-953-51-1590-8, InTech, DOI: 10.5772/58394. - Preparation of stripe-patterned heterogeneous hydrogel sheets using microfluidic devices for high-density coculture of hepatocytes and fibroblasts.
Kobayashi A, Yamakoshi K, Yajima Y, Utoh R, Yamada M, Seki M.
J Biosci Bioeng. 2013 Dec;116(6):761-7. PMID: 23845912 - Metre-long cell-laden microfibres exhibit tissue morphologies and functions.
Onoe H, Okitsu T, Itou A, Kato-Negishi M, Gojo R, Kiriya D, Sato K, Miura S, Iwanaga S, Kuribayashi-Shigetomi K, Matsunaga YT, Shimoyama Y, Takeuchi S
Nat Mater. 2013 Jun;12(6):584-90. PMID: 23542870 - Controlled formation of heterotypic hepatic micro-organoids in anisotropic hydrogel microfibers for long-term preservation of liver-specific functions.
Yamada M, Utoh R, Ohashi K, Tatsumi K, Yamato M, Okano T, Seki M.
Biomaterials. 2012 Nov;33(33):8304-15. PMID: 22906609 - Molding cell beads for rapid construction of macroscopic 3D tissue architecture.
Matsunaga YT, Morimoto Y, Takeuchi S.
Adv Mater. 2011 Mar 25;23(12):H90-4. PMID:21360782 - Evaluation by bone scintigraphy of osteogenic activity of commercial bioceramics (porous beta-TCP and HAp particles) subcutaneously implanted in rats.Nakayama H, Kawase T, Kogami H, Okuda K, Inoue H, Oda T, Hayama K, Tsuchimochi M, Wolff LF.
J Biomater Appl. 2010 May;24(8):751-68. PMID: 19726531 - A dynamic microarray device for paired bead-based analysis.
Teshima T, Ishihara H, Iwai K, Adachi A, Takeuchi S.
Lab Chip. 2010 Sep 21;10(18):2443-8. PMID: 20697655 - The effect of gradually graded shear stress on the morphological integrity of a huvec-seeded compliant small-diameter vascular graft.
Inoguchi H, Tanaka T, Maehara Y, Matsuda T.
Biomaterials. 2007 Jan;28(3):486-95. PMID: 17034847 - Delivery of a growth factor fusion protein having collagen-binding activity to wound tissues.
Ishikawa T, Terai H, Yamamoto T, Harada K, Kitajima T.
Artif Organs. 2003 Feb;27(2):147-54. PMID: 12580771 - Development of composite cultured oral mucosa utilizing collagen sponge matrix and contracted collagen gel: a preliminary study for clinical applications.
Moriyama T, Asahina I, Ishii M, Oda M, Ishii Y, Enomoto S.
Tissue Eng. 2001 Aug;7(4):415-27. PMID: 11506731 - Cytotoxicity of reactive oxygen species and related agents toward undifferentiated and differentiated rat phenochromocytoma PC12 cells.
Sasaki N, Baba N, Matsuo M.
Biol Pharm Bull. 2001 May;24(5):515-519. PMID: 11379772 - Inhibitory effects of human serum on human fetal skin fibroblast migration: migration-inhibitory activity and substances in serum, and its age-related changes.
Kondo H, Yonezawa Y, Ito H.
In Vitro Cell Dev Biol Anim. 2000 Apr;36(4):256-61. PMID: 10852351