SELECTION BY APPLICATION / Sponges and Discs / 3D Mechanical stress cultures
 AteloCell®3D Mechanical stress cultures
AteloCell®3D Mechanical stress cultures
 AteloCell®3D Mechanical stress cultures
AteloCell®3D Mechanical stress cultures
Mechanical stress in the body
Mechanical stress is taking place throughout our daily lives. Examples include stimulation in the oral cavity by chewing or orthodontics, elongation stimulation by respiration, and shear stress due to air currents. Other examples include effects in the cardiovascular system due to shear stress caused by blood flow, or by stretch stimulation due to blood pressure and pulsation, as well as stimulation in the musculoskeletal tissue by daily movement and exercise. Many have heard of the muscle and bone atrophy that affects astronauts after extended stays in space; this has been attributed to the lack of mechanical stress in the microgravity environment.
Evaluation of mechanical stress at the cell level
The cytoskeleton is altered by extension stimulation. To study the phenomena described above at the cellular level, cell culture devices that apply extension stimulation to cells seeded in a silicon chamber are commercially available. Further, it has been reported that repeated compressive stimulation can be applied to cells in 3D culture in collagen gels or in collagen sponges.
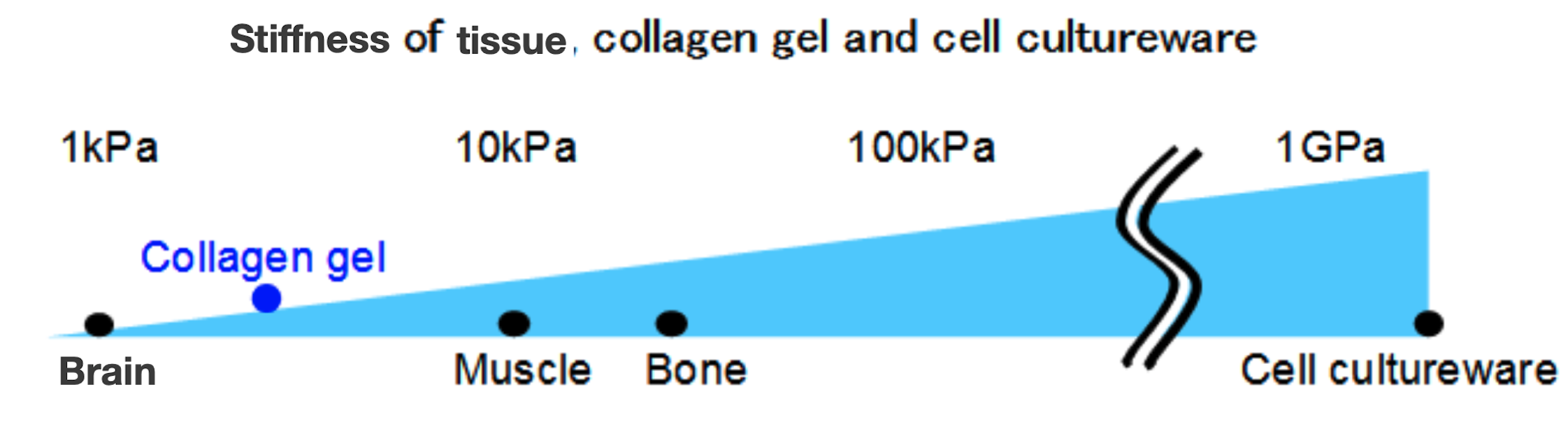
Stiffness of tissues and culture substrates
Cultured cells are affected not only by conditions that apply dynamic mechanical stresses, but also by the hardness of the substrate during cell culture. General plastic and glass labware for cell culturing have a hardness exceeding 1 GPa. In contrast, the in vivo hardness of soft tissues like brain and muscle ranges from 0.1-1.0 kPa and 8-17 kPa, respectively; and 25-40 kPa for hard tissues like bone. Thus, the hardness of conventional cell cultureware and the tissues differ greatly. Therefore, in recent years, there have been reports of cell culture using polyacrylamide gels or collagen gels with different stiffnesses. It also has been suggested that the hardness of the substrate affects the direction of differentiation of stem cells and the evolution of cancer malignancy.
Mechanical stress is taking place throughout our daily lives. Examples include stimulation in the oral cavity by chewing or orthodontics, elongation stimulation by respiration, and shear stress due to air currents. Other examples include effects in the cardiovascular system due to shear stress caused by blood flow, or by stretch stimulation due to blood pressure and pulsation, as well as stimulation in the musculoskeletal tissue by daily movement and exercise. Many have heard of the muscle and bone atrophy that affects astronauts after extended stays in space; this has been attributed to the lack of mechanical stress in the microgravity environment.
Evaluation of mechanical stress at the cell level
The cytoskeleton is altered by extension stimulation. To study the phenomena described above at the cellular level, cell culture devices that apply extension stimulation to cells seeded in a silicon chamber are commercially available. Further, it has been reported that repeated compressive stimulation can be applied to cells in 3D culture in collagen gels or in collagen sponges.
Stiffness of tissues and culture substrates
Cultured cells are affected not only by conditions that apply dynamic mechanical stresses, but also by the hardness of the substrate during cell culture. General plastic and glass labware for cell culturing have a hardness exceeding 1 GPa. In contrast, the in vivo hardness of soft tissues like brain and muscle ranges from 0.1-1.0 kPa and 8-17 kPa, respectively; and 25-40 kPa for hard tissues like bone. Thus, the hardness of conventional cell cultureware and the tissues differ greatly. Therefore, in recent years, there have been reports of cell culture using polyacrylamide gels or collagen gels with different stiffnesses. It also has been suggested that the hardness of the substrate affects the direction of differentiation of stem cells and the evolution of cancer malignancy.

| Product name (click for order info) | Cat no (click for datasheet) | Size | Notes | ||
Atelocollagen sponge Mighty 25pcs (Mighty) |
KOU-CSM-25 | 25 pcs/bottle |  Atelocollagen sponge MIGHTY consists primarily of type I atelocollagen derived from bovine dermis and can withstand compressive loadings of up to 40 kPa. Culturing cells in MIGHTY under cyclic compressive loading simulates the in vivo environment for evaluating cell function. MIGHTY is also useful as a scaffold for 3D culture. |
||
| Collagen Type | Source | Collagenase harvestable | Product form | Specs | Storage |
| Atelo, type 1 | Bovine dermis | Yes | Sterilized, cross-linked sponge | diameter 5mm X height 3mm, pore size: 100-200µm |
RT |
| Product name (click for order info) | Cat no (click for datasheet) | Size | Notes | ||
Atelocollagen sponge Mighty 50pcs (Mighty) |
KOU-CSM-50 | 50 pcs/bottle |  Atelocollagen sponge MIGHTY consists primarily of type I atelocollagen derived from bovine dermis and can withstand compressive loadings of up to 40 kPa. Culturing cells in MIGHTY under cyclic compressive loading simulates the in vivo environment for evaluating cell function. MIGHTY is also useful as a scaffold for 3D culture. |
||
| Collagen Type | Source | Collagenase harvestable | Product form | Specs | Storage |
| Atelo, type 1 | Bovine dermis | Yes | Sterilized, cross-linked sponge | diameter 5mm X height 3mm, pore size: 100-200µm |
RT |
