- CAC Antibody Collection Index Page
Antibody Group
Advanced glycation end products (AGEs)
Autophagy and apoptosis
Bacteria-related
Calcium-binding proteins
Cancer
CD44 for enriching cancer stem cells
Tumor markers
Tumor inhibitors
Chaperones
Cytoskeleton
DNA damage
UV-induced DNA lesions
8-Nitroguanosine for oxidative stress research
Nucleotide excision repair factors
Epigenetics and chromatin
Histone H3 variants
Post-translationally-modified histone H3
Chromatin structure modifiers
Drosophila chromatin
Epitope tags
Exosomes
Extracellular matrix
Glycosaminoglycans (GAGs)
Proteoglycans
Matrix and basement membrane
Cell adhesion and hemidesmosome-related
Bone and cartilage-related
Wound repair-related
Hedgehog pathway
Hormones
Immunology
Fish CD4 and CD8α
Allergic disease-related
Adaptive and innate immunity
Macrophages
Inflammatory cytokines
Viral recognition pathways
Vpr for HIV research
Insulin-like growth factor-related
Mitochondria-related
Neurobiology
Neurodegenerative disease markers
Muscarinic acetylcholine receptors
Miscellaneous
Nuclear import and export
Oxidative stress
Plant-related
Plant hormones
Plant autophagy and apoptosis
Plant stress response
Plant stress response
Proteasomes
Puromycin-specific
Reproductive biology
Small molecules
Stem cells
Novel iPS/ES markers
Pluripotency-associated
Sumoylation pathway
TGF-beta pathway
TGF-beta LAP-d
TGF-beta signaling
Transcription factors
Transporters
Tyrosine phosphatases
Ubiquitin-Proteasome Related
 CAC Antibody Collection
CAC Antibody Collection
The antibodies on this page are part of Cosmo Bio's exclusive CAC Collection. For many many thousands of other antibodies from many different makers, use our Search the Store function and our Explore Products drop down menu.
Proteasomes
Regulating protein stability and turnover is a key task in the cell. Besides lysosomes, ubiquitin‐mediated proteasomal degradation comprises the major proteolytic pathway in eukaryotes. Proteins destined for degradation by the proteasome are conjugated by a ‘tag’, a ubiquitin chain to a lysine, through an extensively regulated enzymatic cascade. The ubiquitylated proteins are subsequently targeted for degradation by the 26S proteasome, the major proteolytic machinery for ubiquitylated proteins in the cell. Ubiquitylation can be considered as another covalent post‐translational modification and signal, comparable to acetylation, glycosylation, methylation, and phosphorylation. However, ubiquitylation has multiple roles in addition to targeting proteins for degradation. Depending on the number of ubiquitin moieties and the linkages made, ubiquitin also plays an important role in DNA repair, protein sorting and virus budding. Unregulated degradation of proteins, or abnormally stable proteins, interfere with several regulatory pathways, and the ubiquitin‐proteasome pathway is affected in a number of diseases, such as neurodegenerative diseases, cellular atrophies and malignancies. Therefore, dissecting the ubiquitin‐proteasome pathway and identifying proteins involved in conjunction with the signals required for specific degradation of certain substrates, would help in developing novel therapeutic approaches to treat diseases where the ubiquitin‐proteasome pathway is impaired. [from: Roos‐Mattjus P. and Sistonen L. The ubiquitin‐proteasome pathway (2009) Annals of Medicine 36(4): 285-295]
| Product name (click for order info) | Cat No (click for datasheet) |
Host | Species specificity |
| Anti 20S Proteasome mAb (Clone GC3α) | CAC-SZU-PS-M01 | MS | YST Fish Frog RT HU Plant |
| Anti 20S Proteasome Subunit Alpha Type-4 mAb (Clone GC3β) | CAC-SZU-PS-M02 | MS | YST Fish Frog RT HU Plant |
| Anti 20S Proteasome Subunit Alpha Type-2 mAb (Clone GC4/5) | CAC-SZU-PS-M03 | MS | YST Fish Frog RT HU Plant |
| Product name | Anti 20S Proteasome mAb (Clone GC3α) |
| Cat No | CAC-SZU-PS-M01 |
| Description | The 26S proteasome is an essential component of the ubiquitin-proteolytic pathway in eukaryotic cells and is responsible for the degradation of most cellular proteins. It is composed of a 20S proteasome catalytic core and regulatory particles at either end. The subunits of the 20S proteasome are classified into two families, α and β. In eukaryotes, the 20S proteasome contains seven α-type subunits and seven β-type subunits. The fourteen subunits are arranged in four rings of seven and form an α7β7β7α7 structure. This antibody recognizes multiple subunits of the 20S proteasome from all organisms tested from yeast to human and is suitable for immunoelectron microscopy. References: 1) Haraguchi, C. M., Mabuchi, T., Hirata, S., Shoda, T., Tokumoto, T., Hoshi, K., Yokota, S. 2007. Possible function of caudal nuclear pocket: degradation of nucleoproteins by ubiquitin-proteasome system in rat spermatids and human sperm. J Histochem Cytochem 55, 585-595. PubMed: 17312012 2) Ohsaki, Y., Cheng, J., Fujita, A., Tokumoto, T., Fujimoto, T. 2006. Cytoplasmic lipid droplets are sites of convergence of proteasomal and autophagic degradation of apolipoprotein B. Mol Biol Cell 17, 2674-2683. PubMed: 16597703 3) Tokumoto, M., Horiguchi, R., Nagahama, Y., Ishikawa, K., Tokumoto, T. 2000. Two proteins, a goldfish 20S proteasome subunit and the protein interacting with 26S proteasome, change in the meiotic cell cycle. Eur J Biochem 267, 97-103. PubMed: 10601855 |
| Host | Mouse |
| Species specificity | YST Fish Frog RT HU Plant |
| Figure 1 | 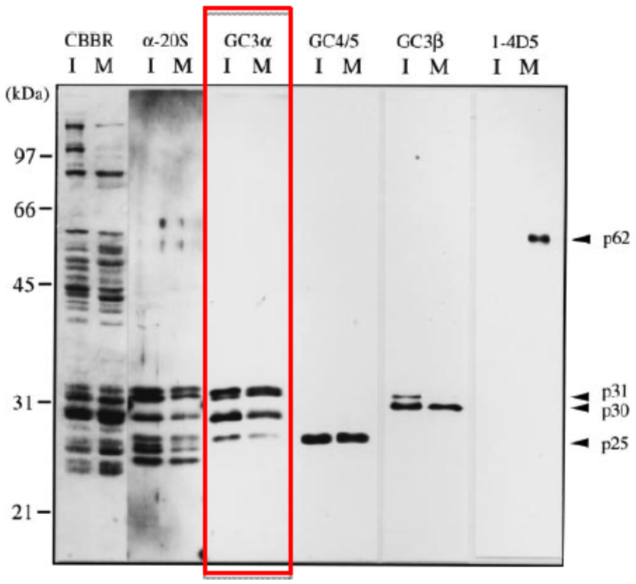 |
| Immunoblotting of purified 26S proteasomes. 26S proteasomes were electrophoresed under denaturing conditions (12.0% gel) and stained with Coomassie Brilliant Blue (CBBR), or, after electroblotting, immunostained with antibodies (α-20S, anti-Xenopus 20S proteasome polyclonal antibodies: GC3α, GC4/5, GC3β or 1-4D5). Lanes I and M indicate 26S proteasomes from immature and mature oocytes, respectively. Protein bands that cross-react with GC4/5 (p25), GC3β (p30 and p31) and 1-4D5 (p62) are indicated. Molecular masses of standard proteins are indicated on the left. |
|
| Figure 2 | 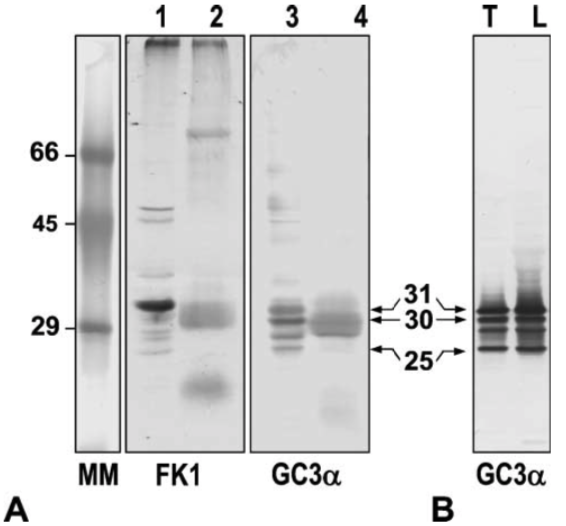 |
| Western blot analysis. (A) Western blot analysis of proteins in rat testicular nuclei (Lanes 1 and 3) and sperm heads (Lanes 2 and 4). Lanes 1 and 2: pUP signals with mouse monoclonal antibody FK1. Lanes 3 and 4: 20S proteasome subunits detected by mouse monoclonal antibody GC3α. Numbers 31, 30, and 25 indicate the molecular mass of each subunit, respectively. MM, molecular mass markers. (B) Western blot analysis of proteasomes partially purified from rat testes (Lane T) and liver (Lane L) using mouse monoclonal antibody to gold fish proteasome subunits (GC3α). |
|
| Figure 3 | 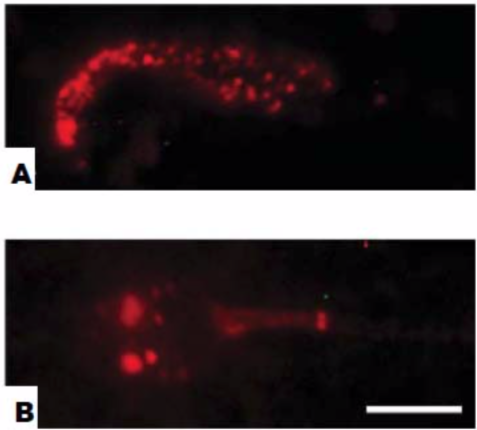 |
| Immunofluorescence staining of rat epididymal sperm and human ejaculated sperm. (A) Smear preparation of rat sperm. (B) Human ejaculated sperm. Bar = 5 um. |
|
| Figure 4 | 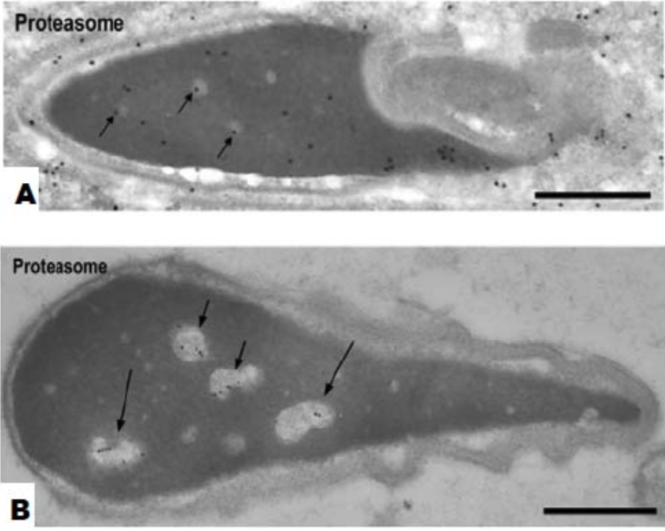 |
| Immunoelectron microscopic localization of proteasomes. (A) Step 19 spermatids. Nuclear staining becomes weaker. Some gold particles are present in clear spots (arrows). Bar = 0.5 um. (B) Nuclei of human ejaculated sperm Gold labeling is observed in the clear spots (short arrows) and in the vacuoles (long arrows) but not in the dense matrix. Bar = 0.5 um. |
|
| Product name | Anti 20S Proteasome Subunit Alpha Type-4 mAb (Clone GC3β) |
| Cat No | CAC-SZU-PS-M02 |
| Description | The 26S proteasome is an essential component of the ubiquitin-proteolytic pathway in eukaryotic cells and is responsible for the degradation of most cellular proteins. It is composed of a 20S proteasome catalytic core and regulatory particles at either end. The subunits of the 20S proteasome are classified into two families, α and β. In eukaryotes, the 20S proteasome contains seven α-type subunits and seven β-type subunits. The fourteen subunits are arranged in four rings of seven and form an α7β7β7α7 structure. This antibody recognizes the α4 subunit of the 20S proteasome from all organisms tested from yeast to human. References: 1) Tokumoto, M., Horiguchi, R., Nagahama, Y., Tokumoto, T. 1999. Identification of the Xenopus 20S proteasome alpha4 subunit which is modified in the meiotic cell cycle. Gene 239, 301-308. PubMed: 10548731 2) Tokumoto, M., Horiguchi, R., Nagahama, Y., Ishikawa, K., Tokumoto, T. 2000. Two proteins, a goldfish 20S proteasome subunit and the protein interacting with 26S proteasome, change in the meiotic cell cycle. Eur J Biochem 267, 97-103. PubMed: 10601855 |
| Host | Mouse |
| Species specificity | YST Fish Frog RT HU Plant |
| Figure 1 | 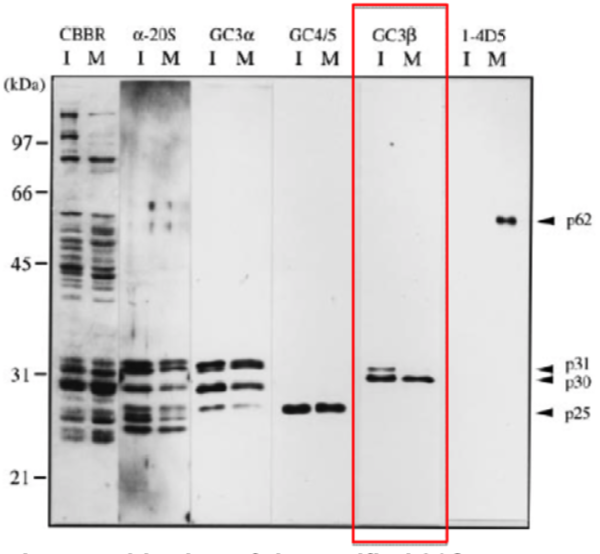 |
| Immunoblotting of purified 26S proteasomes. 26S proteasomes were electrophoresed under denaturing conditions (12.0% gel) and stained with Coomassie Brilliant Blue (CBBR), or, after electroblotting, immunostained with antibodies (α-20S, anti-Xenopus 20S proteasome polyclonal antibodies: GC3α, GC4/5, GC3β or 1-4D5). Lanes I and M indicate 26S proteasomes from immature and mature oocytes, respectively. Protein bands that cross-react with GC4/5 (p25), GC3β (p30 and p31) and 1-4D5 (p62) are indicated. Molecular masses of standard proteins are indicated on the left. |
|
| Product name | Anti 20S proteasome mAb (Clone GC4/5) |
| Cat No | CAC-SZU-PS-M03 |
| Description | The 26S proteasome is an essential component of the ubiquitin-proteolytic pathway in eukaryotic cells and is responsible for the degradation of most cellular proteins. It is composed of a 20S proteasome catalytic core and regulatory particles at either end. The subunits of the 20S proteasome are classified into two families, α and β. In eukaryotes, the 20S proteasome contains seven α-type subunits and seven β-type subunits. The fourteen subunits are arranged in four rings of seven and form an α7β7β7α7 structure. This antibody recognizes α2 subunit of the 20S proteasome from all organisms tested from yeast to human. This antibody is suitable for immunoelectron microscopy. References: 1) Tokumoto, T., Tokumoto, M., Seto, K., Horiguchi, R., Nagahama, Y., Yamada, S., Ishikawa, K., Lohka, M. J. 1999. Disappearance of a novel protein component of the 26S proteasome during Xenopus oocyte maturation. Exp Cell Res 247, 313-319.. PubMed: 10066358 2) Wakata, Y., Tokumoto, M., Horiguchi, R., Ishikawa, K., Nagahama, Y., Tokumoto, T. 2004. Identification of alpha-type subunits of the Xenopus 20S proteasome and analysis of their changes during the meiotic cell cycle. BMC Biochem 5, 18. PubMed: 15603592 3) Tokumoto, M., Horiguchi, R., Nagahama, Y., Ishikawa, K., Tokumoto, T. 2000. Two proteins, a goldfish 20S proteasome subunit and the protein interacting with 26S proteasome, change in the meiotic cell cycle. Eur J Biochem 267, 97-103. PubMed: 10601855 |
| Host | Mouse |
| Species specificity | YST Fish Frog RT HU Plant |
| Figure 1 | 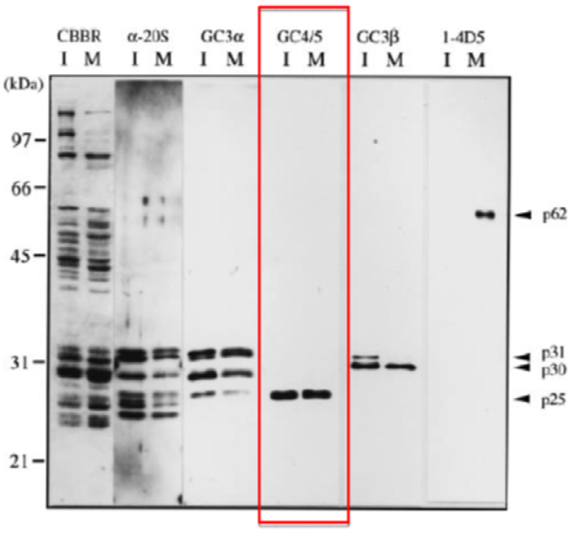 |
| Immunoblotting of purified 26S proteasomes. 26S proteasomes were electrophoresed under denaturing conditions (12.0% gel) and stained with Coomassie Brilliant Blue (CBBR), or, after electroblotting, immunostained with antibodies (α-20S, anti-Xenopus 20S proteasome polyclonal antibodies: GC3α, GC4/5, GC3β or 1-4D5). Lanes I and M indicate 26S proteasomes from immature and mature oocytes, respectively. Protein bands that cross-react with GC4/5 (p25), GC3β (p30 and p31) and 1-4D5 (p62) are indicated. Molecular masses of standard proteins are indicated on the left. |
|
| Figure 2 | 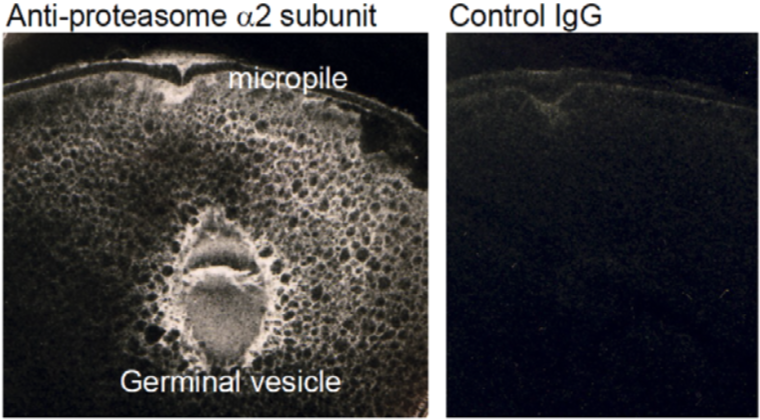 |
| Immunoelectron microscopic localization of proteasome α2 subunit. | |
