 CAC Antibody Collection
CAC Antibody Collection
The antibodies on this page are part of Cosmo Bio's exclusive CAC Collection. For many many thousands of other antibodies from many different makers, use our Search the Store function and our Explore Products drop down menu.
The entry and exit of large molecules from the cell nucleus is tightly controlled by the nuclear pore complexes (NPCs). Although small molecules can enter the nucleus without regulation, macromolecules such as RNA and proteins require association with transport factors known as nuclear transport receptors, like karyopherins called importins to enter the nucleus and exportins to exit.
Protein that must be imported to the nucleus from the cytoplasm carry nuclear localization signals (NLS) that are bound by importins. An NLS is a sequence of amino acids that acts as a tag. They are diverse in their composition and most commonly hydrophilic, although hydrophobic sequences have also been documented. Proteins, transfer RNA, and assembled ribosomal subunits are exported from the nucleus due to association with exportins, which bind signaling sequences called nuclear export signals (NES). The ability of both importins and exportins to transport their cargo is regulated by the small Ras related GTPase, Ran.
GTPases are enzymes that bind to a molecule called guanosine triphosphate (GTP) which they then hydrolyze to create guanosine diphosphate (GDP) and release energy. Ran is in a different conformation depending on whether it is bound to GTP or GDP. In its GTP bound state, Ran is capable of binding karyopherins (importins and exportins). Importins release cargo upon binding to RanGTP, while exportins must bind RanGTP to form a ternary complex with their export cargo. The dominant nucleotide binding state of Ran depends on whether it is located in the nucleus (RanGTP) or the cytoplasm (RanGDP). [from: Wikipedia contributors. (2019, March 27). Nuclear transport. In Wikipedia, The Free Encyclopedia. Retrieved 21:37, June 4, 2019, from https://en.wikipedia.org/w/index.php?title=Nuclear_transport&oldid=889661407]
Protein that must be imported to the nucleus from the cytoplasm carry nuclear localization signals (NLS) that are bound by importins. An NLS is a sequence of amino acids that acts as a tag. They are diverse in their composition and most commonly hydrophilic, although hydrophobic sequences have also been documented. Proteins, transfer RNA, and assembled ribosomal subunits are exported from the nucleus due to association with exportins, which bind signaling sequences called nuclear export signals (NES). The ability of both importins and exportins to transport their cargo is regulated by the small Ras related GTPase, Ran.
GTPases are enzymes that bind to a molecule called guanosine triphosphate (GTP) which they then hydrolyze to create guanosine diphosphate (GDP) and release energy. Ran is in a different conformation depending on whether it is bound to GTP or GDP. In its GTP bound state, Ran is capable of binding karyopherins (importins and exportins). Importins release cargo upon binding to RanGTP, while exportins must bind RanGTP to form a ternary complex with their export cargo. The dominant nucleotide binding state of Ran depends on whether it is located in the nucleus (RanGTP) or the cytoplasm (RanGDP). [from: Wikipedia contributors. (2019, March 27). Nuclear transport. In Wikipedia, The Free Encyclopedia. Retrieved 21:37, June 4, 2019, from https://en.wikipedia.org/w/index.php?title=Nuclear_transport&oldid=889661407]
| Product name (click for order info) | Cat No (click for datasheet) |
Host | Species specificity |
| Anti Importin-4 (IPO4) mAb (Clone 3C2) | CAC-CEC-023 | RT | HU MS RT MKY HAM |
| Anti Exportin-5 (XPO5) mAb (Clone 1D11) | CAC-CEC-024 | RT | HU |
| Anti GTP-Binding Nuclear Protein Ran mAb (Clone 1D6C10) | CAC-CEC-025 | RT | HU MS RT MKY |
| Anti Nuclear Pore Glycoprotein p62 (NUP62) mAb (Clone 8A12) | CAC-CEC-028 | RT | HU MS |
| Anti Nuclear Pore Complex Protein Nup153 (NUP153) mAb (Clone 7F9E12) | CAC-CE-010A | RT | HU |
| Product name | Anti Importin-4 (IPO4) mAb (Clone 3C2) |
| Cat No | CAC-CE-005A |
| Description | Functions in nuclear protein import as nuclear transport receptor. Serves as receptor for nuclear localization signals (NLS) in cargo substrates. Is thought to mediate docking of the importin/substrate complex to the nuclear pore complex (NPC) through binding to nucleoporin and the complex is subsequently translocated through the pore by an energy requiring, Ran-dependent mechanism. At the nucleoplasmic side of the NPC, Ran binds to the importin, the importin/substrate complex dissociates and importin is re-exported from the nucleus to the cytoplasm where GTP hydrolysis releases Ran. The directionality of nuclear import is thought to be conferred by an asymmetric distribution of the GTP- and GDP-bound forms of Ran between the cytoplasm and nucleus (By similarity). Mediates the nuclear import of RPS3A. In vitro, mediates the nuclear import of human cytomegalovirus UL84 by recognizing a non-classical NLS. [from: The UniProt Consortium, UniProt: a worldwide hub of protein knowledge, Nucleic Acids Research, Volume 47, Issue D1, 08 January 2019, Pages D506–D515, https://doi.org/10.1093/nar/gky1049] References: 1) Miyauchi et al. (2005) J. Biol. Chem. 280:40901-40908. 2) Wang et al. (2010) PNAS. 107:16131-16136. |
| Host | RT |
| Species specificity | HU MS RT MKY HAM |
| Figure 1 | 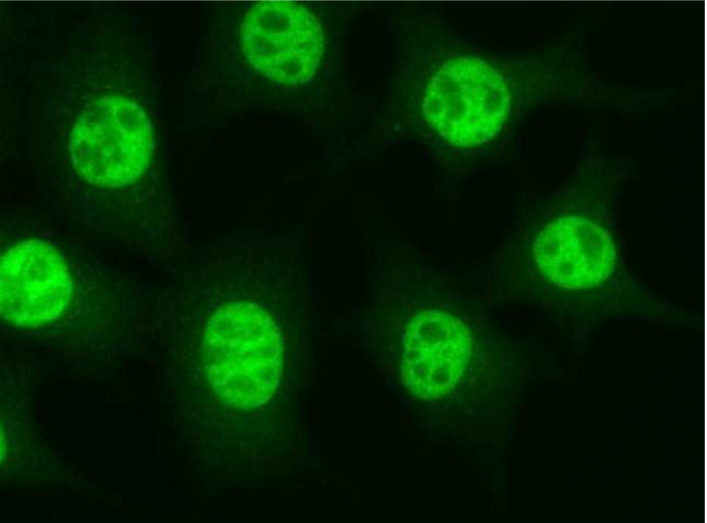 |
| Immunocytochemistry/Immunofluorescence analysis of Importin-4 antibody (3C2) on L929 (mouse) cells. | |
| Figure 2 | 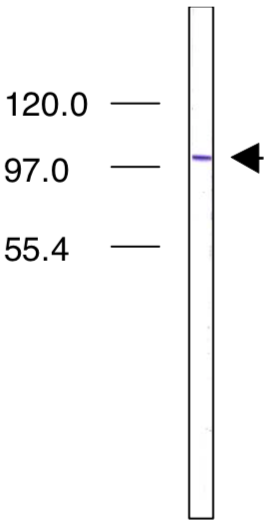 |
| Immunoblot analysis of Importin4 antibody (3C2) on L1210 (mouse) cell extracts. | |
| Product name | Anti Exportin-5 (XPO5) mAb (Clone 1D11) |
| Cat No | CAC-CE-006A |
| Description | Exportin-5 (XPO5) is a protein that, in humans, is encoded by the XPO5 gene.[5][6][7] In eukaryotic cells, the primary purpose of XPO5 is to export pre-microRNA (also known as pre-miRNA) out of the nucleus and into the cytoplasm, for further processing by the Dicer enzyme. Once in the cytoplasm, the microRNA (also known as miRNA) can act as a gene silencer by regulating translation of mRNA. Although XPO5 is primarily involved in the transport of pre-miRNA, it has also been reported to transport tRNA. [from: Wikipedia contributors. (2019, February 11). XPO5. In Wikipedia, The Free Encyclopedia. Retrieved 22:11, June 4, 2019, from https://en.wikipedia.org/w/index.php?title=XPO5&oldid=882799194] References: Katahira and Yoneda (2011) Traffic. 12:1468-1474. |
| Host | RT |
| Species specificity | HU |
| Figure 1 | 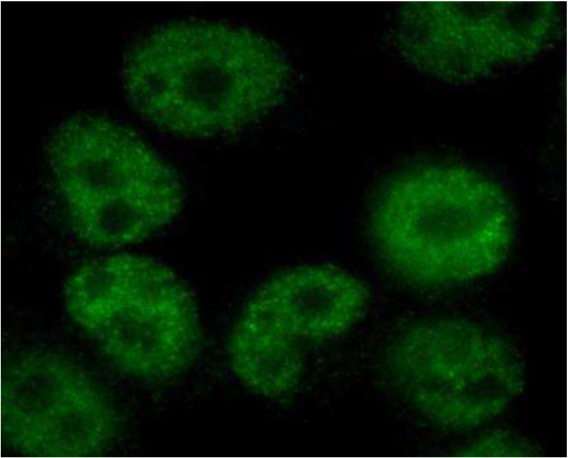 |
| Immunocytochemistry/Immunofluorescence analysis of Exportin-5 antibody (1D11) on HeLa cells. | |
| Figure 2 | 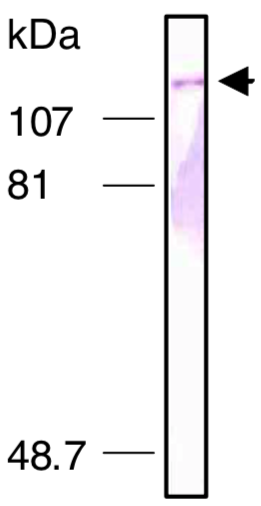 |
| Immunoblot analysis of Exportin-5 antibody (1D11) on HeLa cell nuclear extracts. | |
| Product name | Anti GTP-Binding Nuclear Protein Ran mAb (Clone 1D6C10) |
| Cat No | CAC-CE-007A |
| Description | Ran (RAs-related Nuclear protein) is a small 25 kDa protein involved in transport into and out of the cell nucleus during interphase and is also involved in mitosis. It is a member of the Ras superfamily. Ran is a small G protein that is essential for the translocation of RNA and proteins through the nuclear pore complex. The Ran protein has also been implicated in the control of DNA synthesis and cell cycle progression, as mutations in Ran have been found to disrupt DNA synthesis. Ran exists in the cell in two nucleotide-bound forms: GDP-bound and GTP-bound. RanGDP is converted into RanGTP through the action of RCC1, the nucleotide exchange factor for Ran. RCC1 is also known as RanGEF (Ran Guanine nucleotide Exchange Factor). Ran's intrinsic GTPase-activity is activated through interaction with Ran GTPase activating protein (RanGAP), facilitated by complex formation with Ran-binding protein (RanBP). GTPase-activation leads to the conversion of RanGTP to RanGDP, thus closing the Ran cycle. Ran can diffuse freely within the cell, but because RCC1 and RanGAP are located in different places in the cell, the concentration of RanGTP and RanGDP differs locally as well, creating concentration gradients that act as signals for other cellular processes. RCC1 is bound to chromatin and therefore located inside the nucleus. RanGAP is cytoplasmic in yeast and bound to the nuclear envelope in plants and animals. In mammalian cells, it is SUMO modified and attached to the cytoplasmic side of the nuclear pore complex via interaction with the nucleoporin RanBP2 (Nup358). This difference in location of the accessory proteins in the Ran cycle leads to a high RanGTP to RanGDP ratio inside the nucleus and an inversely low RanGTP to RanGDP ratio outside the nucleus. In addition to a gradient of the nucleotide bound state of Ran, there is a gradient of the protein itself, with a higher concentration of Ran in the nucleus than in the cytoplasm. Cytoplasmic RanGDP is imported into the nucleus by the small protein NTF2 (Nuclear Transport Factor 2), where RCC1 can then catalyze exchange of GDP for GTP on Ran. [from: Wikipedia contributors. (2019, February 22). Ran (protein). In Wikipedia, The Free Encyclopedia. Retrieved 22:23, June 4, 2019, from https://en.wikipedia.org/w/index.php?title=Ran_(protein)&oldid=884595641] References: 1) Güttler and Görlich (2011) EMBO J. 30:3457-3474. |
| Host | RT |
| Species specificity | HU MS RT MKY |
| Figure 1 | 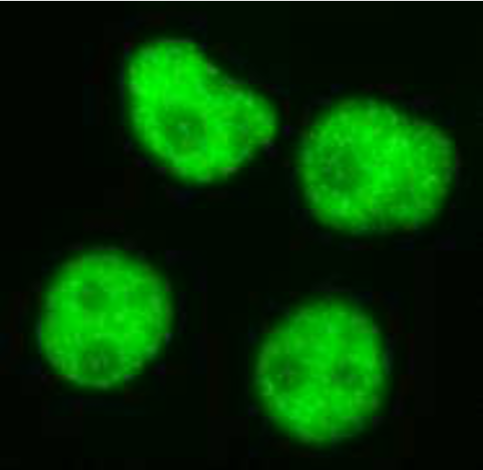 |
| Immunocytochemistry/Immunofluorescence analysis of Ran antibody (1D6C10) on HeLa cells. | |
| Figure 2 | 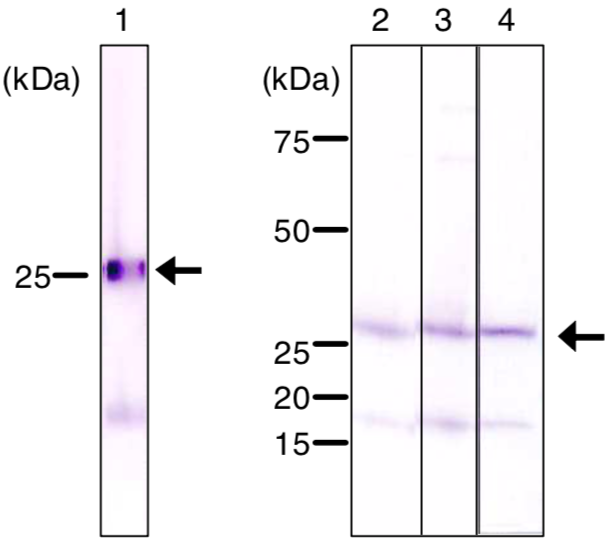 |
| Immunoblot analysis of Ran antibody (1D6C10) on nuclear extracts. 1) HeLa. 2) COS1(simian). 3) L929(mouse). 4) NRK(rat) cell total extracts. |
|
| Product name | Anti Nuclear Pore Glycoprotein p62 (NUP62) mAb (Clone 8A12) |
| Cat No | CAC-CE-008A |
| Description | Nucleoporin p62 (p62) is a protein complex associated with the nuclear envelope. The p62 protein remains associated with the nuclear pore complex-lamina fraction. p62 is synthesized as a soluble cytoplasmic precursor of 61 kDa followed by modification that involve addition of N-acetylglucosamine residues, followed by association with other complex proteins. The nuclear pore complex is a massive structure that extends across the nuclear envelope, forming a gateway that regulates the flow of macromolecules between the nucleus and the cytoplasm. Nucleoporins are the main components of the nuclear pore complex in eukaryotic cells. The protein encoded by this gene is a member of the FG-repeat containing nucleoporins and is localized to the nuclear pore central plug. This protein associates with the importin alpha/beta complex which is involved in the import of proteins containing nuclear localization signals. Multiple transcript variants of this gene encode a single protein isoform. P62 appears to interact with mRNA during transport out of the nucleus. P62 also interacts with a nuclear transport factor (NTF2) protein that is involved in trafficking proteins between cytoplasm and nucleus. Another protein, importin (beta) binds to the helical rod section of p62, which also binds NTF2 suggesting the formation of a higher order gating complex. Karyopherin beta2 (transportin), a riboprotein transporter also interacts with p62. P62 also interacts with Nup93, and when Nup98 is depleted p62 fails to assemble with nuclear pore complexes. Mutant pores could not dock/transport proteins with nuclear localization signals or M9 import signals. [from: Wikipedia contributors. (2019, February 16). Nucleoporin 62. In Wikipedia, The Free Encyclopedia. Retrieved 22:34, June 4, 2019, from https://en.wikipedia.org/w/index.php?title=Nucleoporin_62&oldid=883556059] References: 1) Fukuhara et al. (2006) Hybridoma. 25:51-59. 2) Maeshima et al. (2006) J. Cell Sci. 119:4442-4451. This antibody is used in refs 1 and 2. |
| Host | RT |
| Species specificity | HU MS |
| Figure 1 | 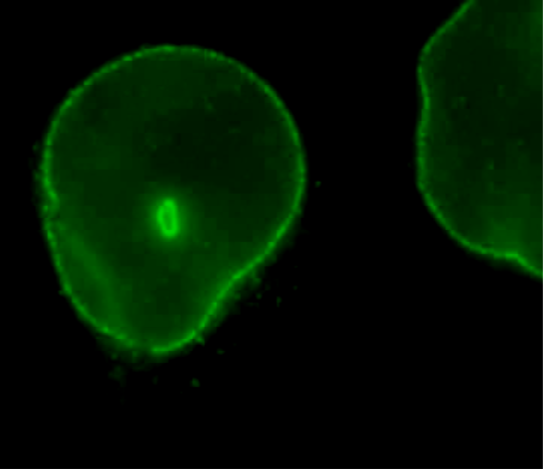 |
| Immunocytochemistry/Immunofluorescence analysis of Nup62/p62 antibody (8A12) on HeLa cells. | |
| Figure 2 | 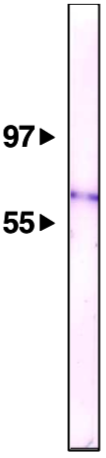 |
| Immunoblot analysis of Nup62/p62 antibody (8A12) on HeLa cell nuclear membrane fractions. | |
| Product name | Anti Nuclear Pore Complex Protein Nup153 (NUP153) mAb (Clone 7F9E12) |
| Cat No | CAC-CE-010A |
| Description | Nucleoporin 153 (Nup153) is a protein which in humans is encoded by the NUP153 gene. It is an essential component of the basket of nuclear pore complexes (NPCs) in vertebrates and required for the anchoring of NPCs. It also acts as the docking site of an importing karyopherin. On the cytoplasmic side of the NPC, Nup358 fulfills an analogous role. Nucleoporin 153 has a mass of 153 kDa (hence its name). It is filamentous and it contains three distinct domains: an N-terminal region within which a pore targeting domain has been identified, a central region containing multiple zinc finger motifs, and a C-terminal region containing multiple XFXFG repeats. [from: Wikipedia contributors. (2019, June 3). Nucleoporin 153. In Wikipedia, The Free Encyclopedia. Retrieved 22:46, June 4, 2019, from https://en.wikipedia.org/w/index.php?title=Nucleoporin_153&oldid=900179284] References: Ball and Ullman (2005) Chromosoma. 114:319-330. |
| Host | RT |
| Species specificity | HU |
| Figure 1 | 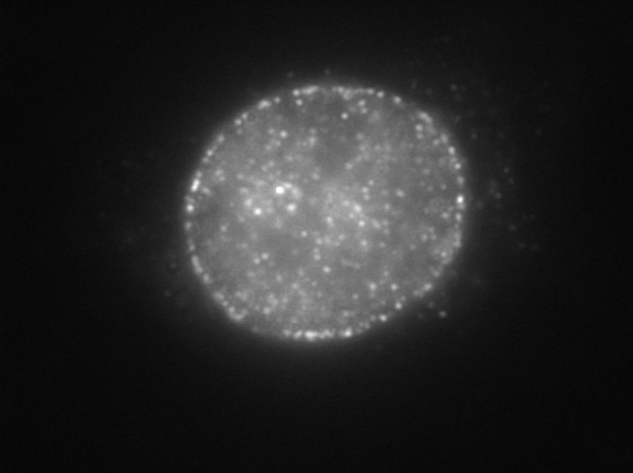 |
| Immunocytochemistry/Immunofluorescence analysis of Nup153 antibody (7F9E12) on HeLa cells. | |
