- CAC Antibody Collection Index Page
Antibody Group
Advanced glycation end products (AGEs)
Autophagy and apoptosis
Bacteria-related
Calcium-binding proteins
Cancer
CD44 for enriching cancer stem cells
Tumor markers
Tumor inhibitors
Chaperones
Cytoskeleton
DNA damage
UV-induced DNA lesions
8-Nitroguanosine for oxidative stress research
Nucleotide excision repair factors
Epigenetics and chromatin
Histone H3 variants
Post-translationally-modified histone H3
Chromatin structure modifiers
Drosophila chromatin
Epitope tags
Exosomes
Extracellular matrix
Glycosaminoglycans (GAGs)
Proteoglycans
Matrix and basement membrane
Cell adhesion and hemidesmosome-related
Bone and cartilage-related
Wound repair-related
Hedgehog pathway
Hormones
Immunology
Fish CD4 and CD8α
Allergic disease-related
Adaptive and innate immunity
Macrophages
Inflammatory cytokines
Viral recognition pathways
Vpr for HIV research
Insulin-like growth factor-related
Mitochondria-related
Neurobiology
Neurodegenerative disease markers
Muscarinic acetylcholine receptors
Miscellaneous
Nuclear import and export
Oxidative stress
Plant-related
Plant hormones
Plant autophagy and apoptosis
Plant stress response
Plant stress response
Proteasomes
Puromycin-specific
Reproductive biology
Small molecules
Stem cells
Novel iPS/ES markers
Pluripotency-associated
Sumoylation pathway
TGF-beta pathway
TGF-beta LAP-d
TGF-beta signaling
Transcription factors
Transporters
Tyrosine phosphatases
Ubiquitin-Proteasome Related
 CAC Antibody Collection
CAC Antibody Collection
The antibodies on this page are part of Cosmo Bio's exclusive CAC Collection. For many many thousands of other antibodies from many different makers, use our Search the Store function and our Explore Products drop down menu.
Fish CD4 and CD8α / Allergic disease-related / Adaptive and innate immunity / Macrophages / Inflammatory cytokines / Viral recognition pathways / Vpr for HIV research
| Immunology: Fish CD4 and CD8α | |||
| Product name (click for order info) | Cat No (click for datasheet) |
Host | Species specificity |
| Anti Fish T-Cell Surface Glycoprotein CD4 mAb (Clone 6D1) | CAC-NIH-NA-01 | RT | Goldfish Carp Zebrafish |
| Anti Fish T-cell Surface Glycoprotein CD8 Alpha Chain (CD8A) mAb (Clone 2C3) | CAC-NIH-NA-02 | RT | Goldfish Carp Zebrafish |
| Immunology: Adaptive and innate immunity | |||
| Product name (click for order info) | Cat No (click for datasheet) |
Host | Species specificity |
| Anti Canine T-Cell Surface Glycoprotein CD3 Epsilon Chain (CD3E) mAb (Clone 1B9-7-1-1) | CAC-ABS-070001 | MS | CAN |
| Anti Feline T-Cell Surface Glycoprotein CD3 Epsilon Chain (CD3E) mAb (Clone 5G-6-7-3) | CAC-ABS-070002 | MS | FEL |
| Anti Canine Neural Cell Adhesion Molecule 1 (NCAM1/CD56) mAb (Clone K9BYU) | CAC-CLI-07001N | MS | CAN |
| Immunology: Macrophages | |||
| Product name (click for order info) | Cat No (click for datasheet) |
Host | Species specificity |
| Anti Human Transmembrane Glycoprotein NMB (GPNMB) pAb (Rabbit, Purified Ig) | CAC-ICA-TG1-RBP1 | RAB | HU |
| Anti Human/Rat Transmembrane Glycoprotein NMB (GPNMB) pAb (Rabbit, Purified Ig) | CAC-ICA-TG1-RBP2 | RAB | HU RT |
| Anti Human/Rat/Mouse Transmembrane Glycoprotein NMB (GPNMB) pAb (Rabbit, Purified Ig) | CAC-ICA-TG1-RBP3 | RAB | HU MS RT |
| Anti Macrophage Scavenger Receptor 1 (MSR1/CD204) mAb (Clone SRA-E5) Sales Discontinued | CAC-KMU-MA01 | MS | HU MS RT BOV RAB POR CAN MKY GP FEL HAM EQ |
| Immunology: Inflammatory cytokines | |||
| Product name (click for order info) | Cat No (click for datasheet) |
Host | Species specificity |
| Anti Interleukin-32 (IL-32) pAb (Rabbit, Antiserum) | CAC-SU-IZ-P02 | RAB | HU |
| Immunology: Viral recognition pathways | |||
| Product name (click for order info) | Cat No (click for datasheet) |
Host | Species specificity |
| Anti Probable ATP-Dependent RNA Helicase DDX58 (RIG-I/RLR-1) mAb (Clone SS1A) | CAC-SU-IZ-M02 | RT | MS |
| Immunology: Vpr for HIV research | |||
| Product name (click for order info) | Cat No (click for datasheet) |
Host | Species specificity |
| Anti HIV-1 Viral protein R (Vpr) mAb (Clone 8D1) | CAC-NCG-M01 | MS | HIV-1 |
| Product name | Anti Fish T-Cell Surface Glycoprotein CD4 mAb (Clone 6D1) |
| Cat No | CAC-NIH-NA-01 |
| Description | This antibody, raised against antigens from Ginbuna carp, permits flow cytometric and immunofluorescence analysis of CD4 helper T cells from zebrafish, goldfish and the cyprinid fish carp. References: 1) Toda H., et al. Conservation of characteristics and functions of CD4 positive lymphocytes in a teleost fish. Dev Comp Immunol. (2011) 35 (6), 650-660. 2) Shibasaki Y., et al. Kinetics of CD4 + and CD8α + T-cell subsets in Graft-Versus-Host Reaction (GVHR) in Gimbuna crucian carp Carassius auratus langsdorfii. |
| Host | Rat |
| Species specificity | Carp Goldfish Zebrafish |
| Figure | 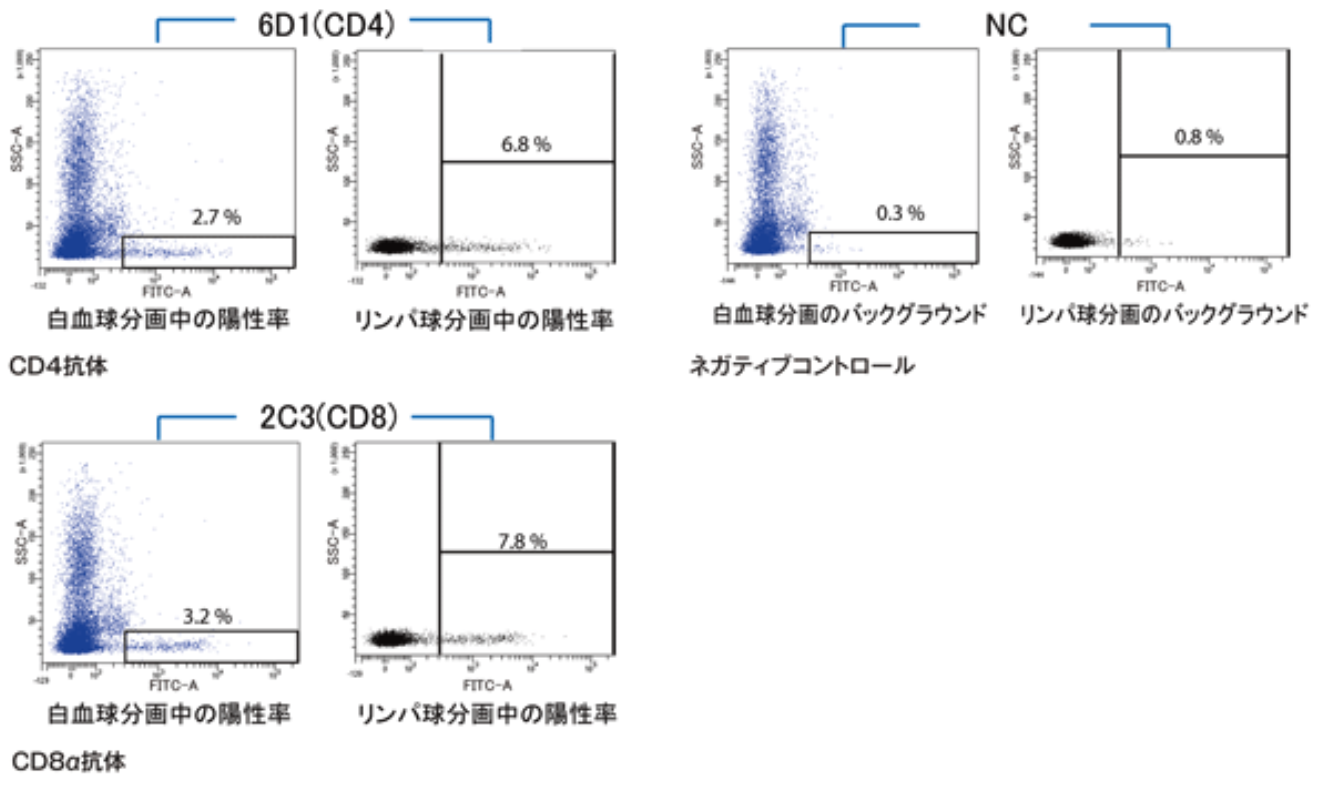 |
| FACS analysis using zebrafish kidney. | |
| Product name | Anti Fish T-cell Surface Glycoprotein CD8 Alpha Chain (CD8A) mAb (Clone 2C3) |
| Cat No | CAC-NIH-NA-02 |
| Description | This antibody, raised against antigens from Ginbuna carp, permits flow cytometric and immunofluorescence analysis of CD8α T cytotoxic cells from zebrafish, goldfish and the cyprinid fish carp. References: 1) Toda H., et al. Allo-antigen specific killing is mediated by CD8 positive T cells in fish. Dev. Comp. Immunol. (2009) 33 (4), 646-652. 2) Shibasaki Y., et al. Kinetics of lymphoid subpopulations in allogeneic grafted scales of ginbuna crucian carp. Dev Comp Immunol. (2015) 52 (1), 75-80. |
| Host | Rat |
| Species specificity | Carp Goldfish Zebrafish |
| Figure |  |
| FACS analysis using zebrafish kidney. | |
| Allergic disease-related (Top) | |
| Allergic diseases represent one of the most common types of disease globally. They incur a substantial global health burden. Major chronic allergic diseases include rhinitis, asthma, atopic dermatitis, gastrointestinal diseases and food allergy. Allergic diseases are complex multifactorial disorders, with genetic, environmental and socioeconomic factors determining disease expression and leading to different phenotypes. Several mechanisms are involved, but many patients suffer from IgE-mediated reactions. However, not all sensitized subjects develop clinical symptoms nor are all individuals with allergic diseases sensitized, suggesting that the relationship between the symptoms of allergy and positive IgE-sensitization is still not clear. Symptoms often begin early in life, but the clinical phenotypes of allergic diseases vary with age, thus increasing their complexity. An important characteristic of complex chronic diseases is their heterogeneity. Heterogeneity of asthma has received increasing attention. On the other hand, allergic diseases tend to aggregate in the same individual as multi-morbidy or else follow an atopic march. | |
| Product name | Anti Serpin B3 (SCCA1/T4-A) mAb (Clone SS6C) |
| Cat No | CAC-SU-IZ-M08 |
| Description |
Squamous cell carcinoma antigen (SCCA) is a member of the ovalbumin family of serine proteinase inhibitors. The protein was isolated from a metastatic cervical squamous cell carcinoma by Kato and Torigoe (1977). SCCA is detected in the superficial and intermediate layers of normal squamous epithelium, whereas the mRNA is detected in the basal and sub-basal levels. The clinical import of SCCA has been as a circulating tumor marker for squamous cell carcinoma, especially those of the cervix, head and neck, lung, and esophagus. Many clinical studies of cervical squamous cell carcinoma show that the percentage of patients with elevated circulating levels of SCCA increases from approximately 12% at stage 0 to more than 90% at stage IV. Levels fall after tumor resection and rise in approximately 90% of the patients with recurrent disease. Similar trends occur in the other types of squamous cell carcinoma, with a maximum sensitivity of approximately 60% for lung, 50% for esophageal, and 55% for head and neck tumors. The neutral form of SCCA (SCCA1, or SERPINB3) is detected in the cytoplasm of normal and some malignant squamous cells, whereas the acidic form (SCCA2, or SERPINB4) is expressed primarily in malignant cells and is the major form found in the plasma of cancer patients. Thus, the appearance of the acidic fraction of SCCA is correlated with more aggressive tumors (summary by Schneider et al., 1995). Gene expression microarray profiling analysis has identified squamous cell cancer antigen (SCCA) as an IL-13 inflammation-induced gene in tracheal epithelial cells and keratinocytes. SCCA expression is increased in asthmatic bronchiale and atopic dermatitis skin. Two isoforms of SCCA are known: SCCA1 and SCCA2. Source: Professor Kenji Dehara, Professor of Molecular Life Science, Faculty of Medicine, Saga University. References: |
| Host | Rat |
| Species specificity | HU |
| Product name | Anti Serpin B4 (Leupin/SCCA2) mAb (Clone SS8J) |
| Cat No | CAC-SU-IZ-M01 |
| Description |
Squamous cell carcinoma antigen (SCCA) is a member of the ovalbumin family of serine proteinase inhibitors. The protein was isolated from a metastatic cervical squamous cell carcinoma by Kato and Torigoe (1977). SCCA is detected in the superficial and intermediate layers of normal squamous epithelium, whereas the mRNA is detected in the basal and sub-basal levels. The clinical import of SCCA has been as a circulating tumor marker for squamous cell carcinoma, especially those of the cervix, head and neck, lung, and esophagus. Many clinical studies of cervical squamous cell carcinoma show that the percentage of patients with elevated circulating levels of SCCA increases from approximately 12% at stage 0 to more than 90% at stage IV. Levels fall after tumor resection and rise in approximately 90% of the patients with recurrent disease. Similar trends occur in the other types of squamous cell carcinoma, with a maximum sensitivity of approximately 60% for lung, 50% for esophageal, and 55% for head and neck tumors. The neutral form of SCCA (SCCA1, or SERPINB3) is detected in the cytoplasm of normal and some malignant squamous cells, whereas the acidic form (SCCA2, or SERPINB4) is expressed primarily in malignant cells and is the major form found in the plasma of cancer patients. Thus, the appearance of the acidic fraction of SCCA is correlated with more aggressive tumors (summary by Schneider et al., 1995). Gene expression microarray profiling analysis has identified squamous cell cancer antigen (SCCA) as an IL-13 inflammation-induced gene in tracheal epithelial cells and keratinocytes. SCCA expression is increased in asthmatic bronchiale and atopic dermatitis skin. Two isoforms of SCCA are known: SCCA1 and SCCA2. Anti-SCCA2 antibody is a rat monoclonal antibody raised against purified E. coli-derived, recombinant human SCCA2. This antibody can be used for the detection of human SCCA2 by immunoprecipitation and ELISA with no cross-reaction to SCCA1. Source: Professor Kenji Dehara, Professor of Molecular Life Science, Faculty of Medicine, Saga University. References: |
| Host | Rat |
| Species specificity | HU |
| Product name | Anti Serpin B3 (SCCA1/T4-A) and Serpin B4 (SCCA2/Leupin) mAb (Clone SS6A) |
| Cat No | CAC-SU-IZ-M07 |
| Description |
Squamous cell carcinoma antigen (SCCA) is a member of the ovalbumin family of serine proteinase inhibitors. The protein was isolated from a metastatic cervical squamous cell carcinoma by Kato and Torigoe (1977). SCCA is detected in the superficial and intermediate layers of normal squamous epithelium, whereas the mRNA is detected in the basal and sub-basal levels. The clinical import of SCCA has been as a circulating tumor marker for squamous cell carcinoma, especially those of the cervix, head and neck, lung, and esophagus. Many clinical studies of cervical squamous cell carcinoma show that the percentage of patients with elevated circulating levels of SCCA increases from approximately 12% at stage 0 to more than 90% at stage IV. Levels fall after tumor resection and rise in approximately 90% of the patients with recurrent disease. Similar trends occur in the other types of squamous cell carcinoma, with a maximum sensitivity of approximately 60% for lung, 50% for esophageal, and 55% for head and neck tumors. The neutral form of SCCA (SCCA1, or SERPINB3) is detected in the cytoplasm of normal and some malignant squamous cells, whereas the acidic form (SCCA2, or SERPINB4) is expressed primarily in malignant cells and is the major form found in the plasma of cancer patients. Thus, the appearance of the acidic fraction of SCCA is correlated with more aggressive tumors (summary by Schneider et al., 1995). Gene expression microarray profiling analysis has identified squamous cell cancer antigen (SCCA) as an IL-13 inflammation-induced gene in tracheal epithelial cells and keratinocytes. SCCA expression is increased in asthmatic bronchiale and atopic dermatitis skin. Two isoforms of SCCA are known: SCCA1 and SCCA2. Anti-SCCA1/2 antibody is a rat monoclonal antibody which obtained from the immunization with purified, E. coli-derived, recombinant human SCCA1. This antibody can be used for the detection of human SCCA1 and SCCA2 by immunoprecipitation and ELISA. References: |
| Host | Rat |
| Species specificity | HU |
| Product name | Anti Serpin B3 (SCCA1/T4-A) and Serpin B4 (SCCA2/Leupin) pAb (Rabbit, Antiserum) |
| Cat No | CAC-SU-IZ-P04 |
| Description |
Squamous cell carcinoma antigen (SCCA) is a member of the ovalbumin family of serine proteinase inhibitors. The protein was isolated from a metastatic cervical squamous cell carcinoma by Kato and Torigoe (1977). SCCA is detected in the superficial and intermediate layers of normal squamous epithelium, whereas the mRNA is detected in the basal and sub-basal levels. The clinical import of SCCA has been as a circulating tumor marker for squamous cell carcinoma, especially those of the cervix, head and neck, lung, and esophagus. Many clinical studies of cervical squamous cell carcinoma show that the percentage of patients with elevated circulating levels of SCCA increases from approximately 12% at stage 0 to more than 90% at stage IV. Levels fall after tumor resection and rise in approximately 90% of the patients with recurrent disease. Similar trends occur in the other types of squamous cell carcinoma, with a maximum sensitivity of approximately 60% for lung, 50% for esophageal, and 55% for head and neck tumors. The neutral form of SCCA (SCCA1, or SERPINB3) is detected in the cytoplasm of normal and some malignant squamous cells, whereas the acidic form (SCCA2, or SERPINB4) is expressed primarily in malignant cells and is the major form found in the plasma of cancer patients. Thus, the appearance of the acidic fraction of SCCA is correlated with more aggressive tumors (summary by Schneider et al., 1995). Gene expression microarray profiling analysis has identified squamous cell cancer antigen (SCCA) as an IL-13 inflammation-induced gene in tracheal epithelial cells and keratinocytes. SCCA expression is increased in asthmatic bronchiale and atopic dermatitis skin. Two isoforms of SCCA are known: SCCA1 and SCCA2. Anti-SCCA antibody is a rabbit polyclonal antibody which obtained from the immunization with purified, E. coli-derived, recombinant human SCCA2. This antibody can be used for the detection of SCCA by immunoblotting and immunostaining. References: |
| Host | Rabbit |
| Species specificity | HU |
| Figure 1 | 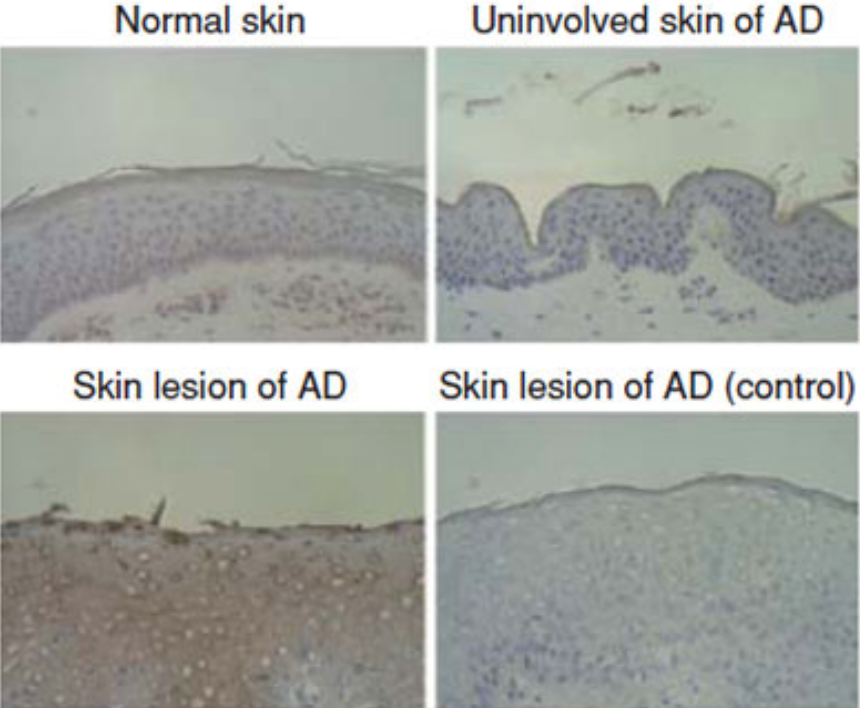 |
| Immunohistochemical staining of squamous cell carcinoma antigen (SCCA) in atopic dermatitis (AD) skin and involved skin of AD patients with or without 200-fold diluted anti-SCCA antibody is depicted. Immunoreacted horseradish peroxidase was developed by diamino benzidine (brown color). | |
| Product name | Anti Squamous Cell Carcinoma Antigen 2 (SERPINB3A/SQN-5) pAb (Rabbit, Antiserum) |
| Cat No | CAC-SU-IZ-P03 |
| Description |
Squamous cell carcinoma antigen (SCCA) is a member of the ovalbumin family of serine proteinase inhibitors. The protein was isolated from a metastatic cervical squamous cell carcinoma by Kato and Torigoe (1977). SCCA is detected in the superficial and intermediate layers of normal squamous epithelium, whereas the mRNA is detected in the basal and sub-basal levels. The clinical import of SCCA has been as a circulating tumor marker for squamous cell carcinoma, especially those of the cervix, head and neck, lung, and esophagus. Many clinical studies of cervical squamous cell carcinoma show that the percentage of patients with elevated circulating levels of SCCA increases from approximately 12% at stage 0 to more than 90% at stage IV. Levels fall after tumor resection and rise in approximately 90% of the patients with recurrent disease. Similar trends occur in the other types of squamous cell carcinoma, with a maximum sensitivity of approximately 60% for lung, 50% for esophageal, and 55% for head and neck tumors. The neutral form of SCCA (SCCA1, or SERPINB3) is detected in the cytoplasm of normal and some malignant squamous cells, whereas the acidic form (SCCA2, or SERPINB4) is expressed primarily in malignant cells and is the major form found in the plasma of cancer patients. Thus, the appearance of the acidic fraction of SCCA is correlated with more aggressive tumors (summary by Schneider et al., 1995). Ray et al. (2005) stated that mouse Serpinb3a, or Scca2, exhibits the cysteine-like serine protease inhibitory function of human SCCA1 and the trypsin-like serine protease inhibitory function of human SCCA2. Thus, Scca2 is the mouse ortholog of both human SCCA1 and SCCA2. Ray et al. (2005) found that challenge of uteroglobin (UGB, or SCGB1A1)-knockout mice with chicken ovalbumin (OVA) resulted in elevated lung expression of Scca2, as well as elevated levels of the cytokines Il4 and Il13 in lung and exacerbated airway inflammation. These effects were countered by reintroduction of recombinant Ugb. Treatment of cultured human bronchial epithelial cells with IL4 or IL13 stimulated SCCA1 and SCCA2 expression via phosphorylation of the transcription factors STAT1 and STAT6. SCCA1 and SCCA2 expression was not upregulated by IL4 or IL13 in the presence of an inhibitor of tyrosine phosphorylation. Ray et al. (2005) proposed that UGB controls allergic asthma by downregulating signaling through IL4 and IL13 and inhibiting SCCA1 and SCCA2 expression. Anti-Serpinb3a antibody is a rabbit polyclonal antibody obtained from immunization with purified E. coli-derived recombinant mouse Serpinb3a. This antibody can be used for the detection of Serpinb3a by immunoblotting and immunostaining. References: |
| Host | Rabbit |
| Species specificity | MS |
| Figure 1 | 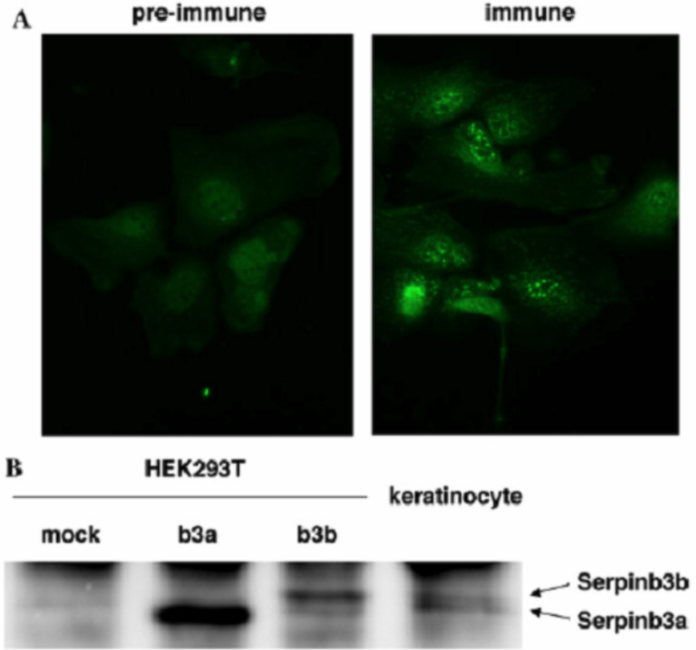 |
| Production and secretion of Serpinb3a and Serpinb3b from mouse keratinocytes. (A) Immunostaining of SCCA-related molecules in mouse keratinocytes by anti-Serpinb3a serum or pre-immune serum is shown. (B) Culture media were prepared from Serpinb3a- or Serpinb3b-transfected HEK293T cells or mouse keratinocytes, and then immunoprecipitates of anti-Serpin3a serum were subjected to Western blotting using anti-Serpinb3a serum. |
|
| Product name | Anti Interleukin-13 Receptor Subunit Alpha-1 (IL-13Ra) D1 Domain mAb (Clone SS4B) |
| Cat No | CAC-SU-IZ-M03 |
| Description | The interleukin-13 receptor is a type I cytokine receptor, binding Interleukin-13. It consists of two subunits, encoded by IL13RA1 and IL4R, respectively. These two genes encode the proteins IL-13Rα1 and IL-4Rα. These form a dimer with IL-13 binding to the IL-13Rα1 chain and IL-4Rα stabilises this interaction. This IL-13 receptor can also instigate IL-4 signaling. In both cases this occurs via activation of the Janus kinase (JAK)/Signal Transducer and Activator of Transcription (STAT) pathway, resulting in phosphorylation of STAT6. Phosphorylated STAT6 dimerises and acts as a transcription factor activating many genes, such as eotaxin. |
| Host | Rat |
| Species specificity | HU |
| Product name | Anti Interleukin-13 Receptor Subunit Alpha-1 (IL-13Ra) D1 Domain mAb (Clone SS4H) |
| Cat No | CAC-SU-IZ-M04 |
| Description | Anti-IL-13Rα1 D1 domain antibody is a rat monoclonal antibody which obtained from the primary immunization with purified, E. coli-derived, recombinant human IL-13Rα1 D1 domain and the secondary immunization with purified, Drosophila S2 cell-derived, recombinant human IL-13Rα1 D1 domain. This antibody can be used for the detection of human IL-13Rα1 D1 domain by flow cytometry. |
| Host | Rat |
| Species specificity | HU |
| Product name | Anti Interleukin-13 Receptor Subunit Alpha-1 (IL-13Ra) mAb (Clone SS12B) |
| Cat No | CAC-SU-IZ-M05 |
| Description | Anti-IL-13Rα1 domain antibody is a mouse monoclonal antibody which obtained from the immunization with purified, silkworm-derived, recombinant human IL-13Rα1 domain. This antibody can be used for the detection of human IL-13Rα1 domain by immunoprecipitation and flow cytometry. |
| Host | Rat |
| Species specificity | HU |
| Adaptive and innate immunity (Top) | |
| The immune system is a host defense system comprising many biological structures and processes within an organism that protects against disease. To function properly, an immune system must detect a wide variety of agents, known as pathogens, from viruses to parasitic worms, and distinguish them from the organism's own healthy tissue. In many species, the immune system can be classified into subsystems, such as the innate immune system versus the adaptive immune system, or humoral immunity versus cell-mediated immunity. In humans, the blood–brain barrier, blood–cerebrospinal fluid barrier, and similar fluid–brain barriers separate the peripheral immune system from the neuroimmune system, which protects the brain. Pathogens can rapidly evolve and adapt, and thereby avoid detection and neutralization by the immune system; however, multiple defense mechanisms have also evolved to recognize and neutralize pathogens. Even simple unicellular organisms such as bacteria possess a rudimentary immune system in the form of enzymes that protect against bacteriophage infections. Other basic immune mechanisms evolved in ancient eukaryotes and remain in their modern descendants, such as plants and invertebrates. These mechanisms include phagocytosis, antimicrobial peptides called defensins, and the complement system. Jawed vertebrates, including humans, have even more sophisticated defense mechanisms, including the ability to adapt over time to recognize specific pathogens more efficiently. Adaptive (or acquired) immunity creates immunological memory after an initial response to a specific pathogen, leading to an enhanced response to subsequent encounters with that same pathogen. This process of acquired immunity is the basis of vaccination. Disorders of the immune system can result in autoimmune diseases, inflammatory diseases and cancer. Immunodeficiency occurs when the immune system is less active than normal, resulting in recurring and life-threatening infections. In humans, immunodeficiency can either be the result of a genetic disease such as severe combined immunodeficiency, acquired conditions such as HIV/AIDS, or the use of immunosuppressive medication. In contrast, autoimmunity results from a hyperactive immune system attacking normal tissues as if they were foreign organisms. Common autoimmune diseases include Hashimoto's thyroiditis, rheumatoid arthritis, diabetes mellitus type 1, and systemic lupus erythematosus. Immunology covers the study of all aspects of the immune system. [from: Wikipedia contributors. (2019, June 1). Immune system. In Wikipedia, The Free Encyclopedia. Retrieved 21:26, June 3, 2019, from https://en.wikipedia.org/w/index.php?title=Immune_system&oldid=899819423] |
|
| Product name | Anti Canine T-Cell Surface Glycoprotein CD3 Epsilon Chain (CD3E) mAb (Clone 1B9-7-1-1) |
| Cat No | CAC-ABS-070001 |
| Description | The CD3-epsilon polypeptide, which together with CD3-gamma, -delta and -zeta, and the T-cell receptor alpha/beta and gamma/delta heterodimers, forms the T cell receptor-CD3 complex. This complex plays an important role in coupling antigen recognition to several intracellular signal-transduction pathways. The genes encoding the epsilon, gamma and delta polypeptides are located in the same cluster on chromosome 11. The epsilon polypeptide plays an essential role in T-cell development. [from: Wikipedia contributors. (2018, October 25). T-cell surface glycoprotein CD3 epsilon chain. In Wikipedia, The Free Encyclopedia. Retrieved 21:31, June 3, 2019, from https://en.wikipedia.org/w/index.php?title=T-cell_surface_glycoprotein_CD3_epsilon_chain&oldid=865624525] |
| Host | MS |
| Species specificity | CAN |
| Figure 1 | 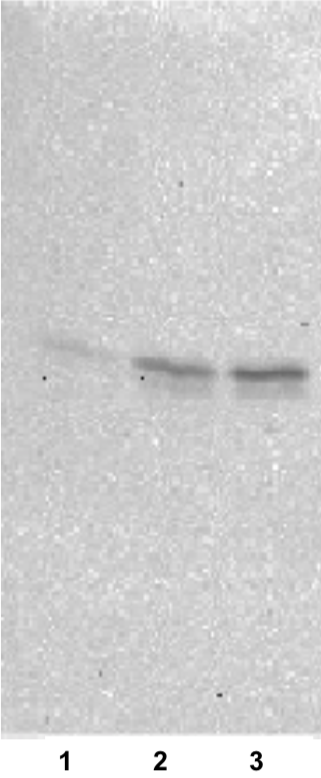 |
| Immunoblot analysis. 1) Peripheral blood mononuclear cells (PBMCs) 2) Anti-canine CD3 antibody-stimulated lymphocytes 3) PHA-stimulated lymphocytes |
|
| Product name | Anti Feline T-Cell Surface Glycoprotein CD3 Epsilon Chain (CD3E) mAb (Clone 5G-6-7-3) |
| Cat No | CAC-ABS-070002 |
| Description | The CD3-epsilon polypeptide, which together with CD3-gamma, -delta and -zeta, and the T-cell receptor alpha/beta and gamma/delta heterodimers, forms the T cell receptor-CD3 complex. This complex plays an important role in coupling antigen recognition to several intracellular signal-transduction pathways. The genes encoding the epsilon, gamma and delta polypeptides are located in the same cluster on chromosome 11. The epsilon polypeptide plays an essential role in T-cell development. [from: Wikipedia contributors. (2018, October 25). T-cell surface glycoprotein CD3 epsilon chain. In Wikipedia, The Free Encyclopedia. Retrieved 21:31, June 3, 2019, from https://en.wikipedia.org/w/index.php?title=T-cell_surface_glycoprotein_CD3_epsilon_chain&oldid=865624525] |
| Host | MS |
| Species specificity | FEL |
| Figure 1 | 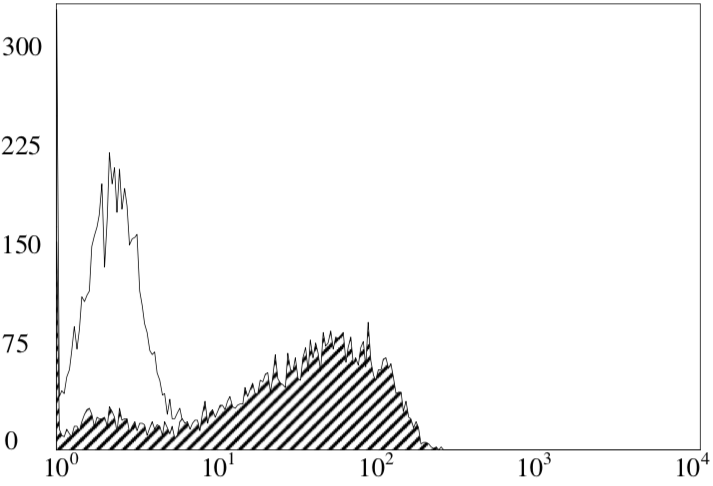 |
| FACS analysis. Clone 5G-6-7-3 staining of feline peripheral blood mononuclear cells (PBMCs). |
|
| Product name | Anti Canine Neural Cell Adhesion Molecule 1 (NCAM1/CD56) mAb (Clone K9BYU) |
| Cat No | CAC-CLI-07001N |
| Description | Neural cell adhesion molecule (NCAM), also called CD56, is a homophilic binding glycoprotein expressed on the surface of neurons, glia and skeletal muscle. Although CD56 is often considered a marker of neural lineage commitment due to its discovery site, CD56 expression is also found in, among others, the hematopoietic system. Here, the expression of CD56 is most stringently associated with, but certainly not limited to, natural killer cells. CD56 has been detected on other lymphoid cells, including gamma delta (γδ) Τ cells and activated CD8+ T cells, as well as on dendritic cells. NCAM has been implicated as having a role in cell–cell adhesion, neurite outgrowth, synaptic plasticity, and learning and memory. During hematopoiesis, CD56 is the prototypic marker of NK cells, also present on subset of CD4+ T cells and CD8+ T cells. [from: Wikipedia contributors. (2019, May 24). Neural cell adhesion molecule. In Wikipedia, The Free Encyclopedia. Retrieved 16:41, June 4, 2019, from https://en.wikipedia.org/w/index.php?title=Neural_cell_adhesion_molecule&oldid=898602503] References: 1) Koike A., Uematsu Y., Bonkobara M., Yamaguchi T., Washizu T., Arai T. (2007) 144th Annual Meeting of Japanese Society of Veterinary Science, I-39. 2) Uematsu Y, Yamaguchi T, Koike A, Yagihara H, Hasegawa D, Matsuki N, Ono K, Washizu T, Arai T, Bonkobara M. (2008) Generation of Monoclonal Antibody against Canine Neural-Cell Adhesion Molecule. Journal of Veterinary Medical Science. 70(8):845-847. |
| Host | MS |
| Species specificity | CAN |
| Figure 1 | 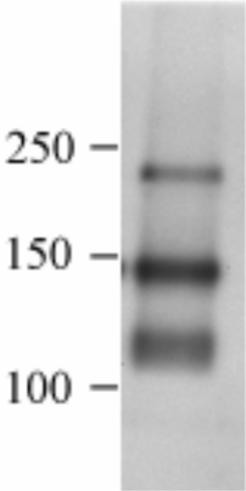 |
| Immunoblot analysis of canine brain tissue with anti-canine CD56 mAb (K9BYU). | |
| Macrophages (Top) | |
| Macrophages were first discovered by Élie Metchnikoff, a Russian zoologist, in 1884. They are a type of white blood cell of the immune system that engulfs and digests cellular debris, foreign substances, microbes, cancer cells, and anything else that does not have the type of proteins specific to healthy body cells on its surface in a process called phagocytosis. These large phagocytes are found in essentially all tissues, where they patrol for potential pathogens by amoeboid movement. They take various forms (with various names) throughout the body (e.g., histiocytes, Kupffer cells, alveolar macrophages, microglia, and others), but all are part of the mononuclear phagocyte system. Besides phagocytosis, they play a critical role in nonspecific defense (innate immunity) and also help initiate specific defense mechanisms (adaptive immunity) by recruiting other immune cells such as lymphocytes. For example, they are important as antigen presenters to T cells. In humans, dysfunctional macrophages cause severe diseases such as chronic granulomatous disease that result in frequent infections. Beyond increasing inflammation and stimulating the immune system, macrophages also play an important anti-inflammatory role and can decrease immune reactions through the release of cytokines. Macrophages that encourage inflammation are called M1 macrophages, whereas those that decrease inflammation and encourage tissue repair are called M2 macrophages. This difference is reflected in their metabolism; M1 macrophages have the unique ability to metabolize arginine to the "killer" molecule nitric oxide, whereas rodent M2 macrophages have the unique ability to metabolize arginine to the "repair" molecule ornithine. However, this dichotomy has been recently questioned as further complexity has been discovered. Human macrophages are about 21 micrometers in diameter and are produced by the differentiation of monocytes in tissues. They can be identified using flow cytometry or immunohistochemical staining by their specific expression of proteins such as CD14, CD40, CD11b, CD64, F4/80 (mice)/EMR1 (human), lysozyme M, MAC-1/MAC-3 and CD68. | |
| Product name | Anti Macrophage Scavenger Receptor 1 (MSR1/CD204) mAb (Clone SRA-E5) |
| Cat No | CAC-KMU-MA01 |
| Description | CD204, also referred as macrophage scavenger receptor class A type I/II (MSR-A) or macrophage scavenger receptor type I/II (MSR1), is one of the major receptors of macrophages and plays important roles in many macrophage-related disorders. CD204 binds various negatively charged macromolecules, such as modified low-density lipoproteins, fucoidan, advanced glycation end-products, denatured collagen, and bacterial components. References: 1) Nature. 1997 Mar 20;386(6622):292-6. 2) Atherosclerosis. 2002 Mar;161(1):123-32. (IHC) 3) J Atheroscler Thromb. 2016;23(1):10-7 (Western blot) 4) Arthritis Res Ther. 2010;12(4):R128. (Flow cytometry) |
| Host | Mouse |
| Species specificity | HU MS RT BOV RAB POR CAN MKY GP FEL HAM EQ |
| Figure 1 | 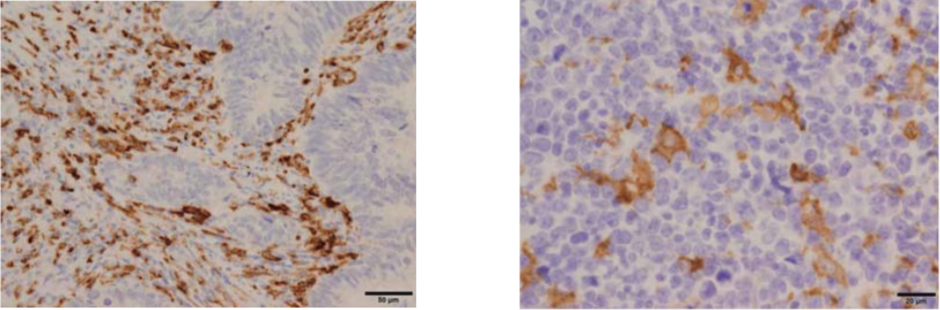 |
| Immunohistochemistry of CD204 in human colon cancer and lymphoma (Paraffin sections). There are many CD204-positive macrophages in stroma of colon cancer (left) and lymphoma (right). Antigen retrieval: 1mM EDTA buffer (pH8.0), heat (microwave for 5 min) |
|
| Figure 2 | 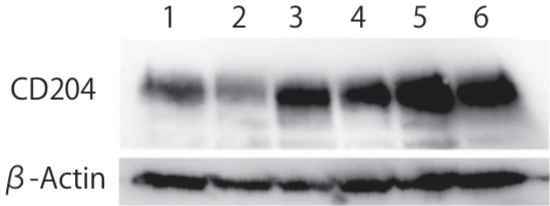 |
| Western blot analysis of CD204 in human monocyte-derived macrophages. Predicted molecular weight of CD204 is 43 kDa, however, molecular mass is approximately 50-70 kDa due to glycosylation under reducing condition. Pre-treatment with N-glycosidase is recommended. Lane 1: Control human monocyte-derived macrophages Lane 2: Macrophages stimulated with IL-4, Lane 3: Macrophages stimulated with IL-10 Lane 4-6: Macrophages stimulated with conditional medium of breast cancer cell lines. |
|
| Product name | Anti Human Transmembrane Glycoprotein NMB (GPNMB) pAb (Rabbit, Purified Ig) |
| Cat No | CAC-ICA-TG1-RBP1 |
| Description |
Originally described in melanoma cells, glycoprotein nonmetastatic melanoma B (Gpnmb), also known as osteoactivin and dendritic cell-associated heparin sulfate proteoglycan-dependent integrin ligand, is a heavily N-glycosylated type I transmembrane domain protein with a short cytoplasmic domain containing an endosomal sorting motif. Gpnmb is expressed in numerous cell types, including dendritic cells, macrophages, retinal pigment epithelial cells, osteoblasts and osteoclasts. Recent studies have demonstrated that Gpnmb expressed in macrophages functions as a feedback regulator of proinflammatory responses, and that binding of Gpnmb on antigen-presenting cells to syndecan-4 on activated T cells inhibits T-cell activation. Thus, Gpnmb expressed on antigen-presenting cells may negatively regulate inflammation. Additionally, Gpnmb is involved in osteoblast maturation, and is also associated with motor neuron survival. We previously isolated the gene encoding Gpnmb and demonstrated that it was differentially expressed in the livers of rats fed a choline-deficient, L-amino acid-defined diet. We also found that transgenic Gpnmb expression attenuates the development of hepatic fibrosis. Furthermore, a recent report has demonstrated that Gpnmb is expressed in macrophages infiltrating into injured liver tissues. These findings suggest a pathophysiologic role for Gpnmb in various disorders, including liver injury. However, the specific nature of Gpnmb involvement in liver injury remains unknown. In this study, we focused on the role of infiltrating hepatic macrophages, especially those expressing Gpnmb, during the repair process in injured livers in a mouse model of acute liver injury. [from: Kumagai K, Tabu K, Sasaki F, Takami Y, Morinaga Y, Mawatari S, et al. (2015) Glycoprotein Nonmetastatic Melanoma B (Gpnmb)-Positive Macrophages Contribute to the Balance between Fibrosis and Fibrolysis during the Repair of Acute Liver Injury in Mice. PLoS ONE 10(11): e0143413. https://doi.org/10.1371/journal.pone.0143413] Lipid-laden macrophages may orchestrate pathology, an accepted notion for inborn lysosomal storage disorders (LSDs) and more recently for metabolic syndrome. The development of enzyme replacement therapy (ERT) for specific LSDs has led in the last decades to the identification of markers of lipid-laden macrophages. In LSDs characterized by foamy macrophages as storage cells, plasma GPNMB has been shown to accurately reflect disease burden. Moreover, GPNMB is also applicable in mouse models of LSDs like Gaucher disease and Niemann-Pick type C. GPNMB is also increased in several acquired diseases, such as metabolic syndrome and neurodegeneration. It therefore might be that these disease conditions share pathophysiological elements, in particular the accumulation of foamy, lysosomal stressed, macrophages. GPNMB is among the most upregulated proteins in lipid-laden macrophages. Nevertheless, at present its exact function in foamy macrophage remains largely enigmatic. Important unanswered questions concern the function(s) served by GPNMB, either the cellular membrane-bound or (extracellular) soluble isoforms, in lipid-laden macrophages and beyond. |
| Host | Rabbit |
| Species specificity | HU |
| Figure 1 | 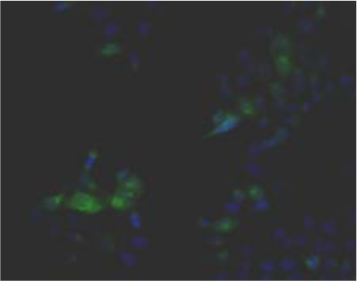 |
| Immunohistochemistry. Hela cells ICAFectin441-transfected with a plasmid encoding human GPNMB were fixed, permeabilized and stained with rabbit anti-human GPNMB polyclonal IgG antibody (diluted 1/100) and Alexa488 goat anti rabbit IgG secondary antibody. Nuclei were counterstained with DAPI. |
|
| Figure 2 | 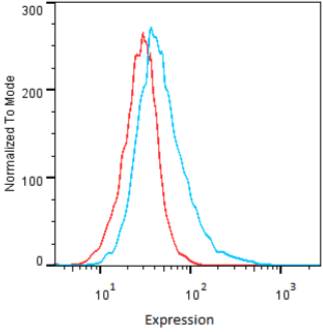 |
| Flow Cytometry. HeLa cells untransfected (red) and ICAFectin441-transfected with a plasmid encoding human GPNMB (blue) were stained with rabbit anti-human GPNMB polyclonal IgG antibody (diluted 1/100) and R-PE goat anti rabbit IgG secondary antibody. |
|
| Figure 3 | 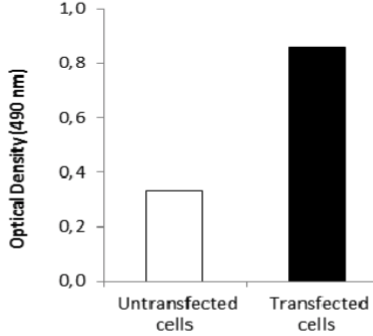 |
| ELISA. Lysates of HeLa cells untransfected (red) and ICAFectin441-transfected with a plasmid encoding human GPNMB were coated in 96 wells plate. Wells were stained with anti-human GPNMB polyclonal IgG and HRP goat anti rabbit IgG secondary antibody. |
|
| Product name | Anti Human/Rat Transmembrane Glycoprotein NMB (GPNMB) pAb (Rabbit, Purified Ig) |
| Cat No | CAC-ICA-TG1-RBP2 |
| Description |
Originally described in melanoma cells, glycoprotein nonmetastatic melanoma B (Gpnmb), also known as osteoactivin and dendritic cell-associated heparin sulfate proteoglycan-dependent integrin ligand, is a heavily N-glycosylated type I transmembrane domain protein with a short cytoplasmic domain containing an endosomal sorting motif. Gpnmb is expressed in numerous cell types, including dendritic cells, macrophages, retinal pigment epithelial cells, osteoblasts and osteoclasts. Recent studies have demonstrated that Gpnmb expressed in macrophages functions as a feedback regulator of proinflammatory responses, and that binding of Gpnmb on antigen-presenting cells to syndecan-4 on activated T cells inhibits T-cell activation. Thus, Gpnmb expressed on antigen-presenting cells may negatively regulate inflammation. Additionally, Gpnmb is involved in osteoblast maturation, and is also associated with motor neuron survival. We previously isolated the gene encoding Gpnmb and demonstrated that it was differentially expressed in the livers of rats fed a choline-deficient, L-amino acid-defined diet. We also found that transgenic Gpnmb expression attenuates the development of hepatic fibrosis. Furthermore, a recent report has demonstrated that Gpnmb is expressed in macrophages infiltrating into injured liver tissues. These findings suggest a pathophysiologic role for Gpnmb in various disorders, including liver injury. However, the specific nature of Gpnmb involvement in liver injury remains unknown. In this study, we focused on the role of infiltrating hepatic macrophages, especially those expressing Gpnmb, during the repair process in injured livers in a mouse model of acute liver injury. [from: Kumagai K, Tabu K, Sasaki F, Takami Y, Morinaga Y, Mawatari S, et al. (2015) Glycoprotein Nonmetastatic Melanoma B (Gpnmb)-Positive Macrophages Contribute to the Balance between Fibrosis and Fibrolysis during the Repair of Acute Liver Injury in Mice. PLoS ONE 10(11): e0143413. https://doi.org/10.1371/journal.pone.0143413] Lipid-laden macrophages may orchestrate pathology, an accepted notion for inborn lysosomal storage disorders (LSDs) and more recently for metabolic syndrome. The development of enzyme replacement therapy (ERT) for specific LSDs has led in the last decades to the identification of markers of lipid-laden macrophages. In LSDs characterized by foamy macrophages as storage cells, plasma GPNMB has been shown to accurately reflect disease burden. Moreover, GPNMB is also applicable in mouse models of LSDs like Gaucher disease and Niemann-Pick type C. GPNMB is also increased in several acquired diseases, such as metabolic syndrome and neurodegeneration. It therefore might be that these disease conditions share pathophysiological elements, in particular the accumulation of foamy, lysosomal stressed, macrophages. GPNMB is among the most upregulated proteins in lipid-laden macrophages. Nevertheless, at present its exact function in foamy macrophage remains largely enigmatic. Important unanswered questions concern the function(s) served by GPNMB, either the cellular membrane-bound or (extracellular) soluble isoforms, in lipid-laden macrophages and beyond. |
| Host | Rabbit |
| Species specificity | HU RT |
| Figure 1 | 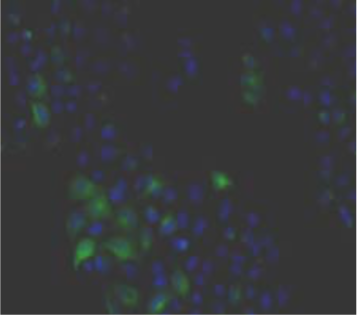 |
| Immunohistochemistry. Hela cells ICAFectin441-transfected with a plasmid encoding human GPNMB were fixed, permeabilized and stained with rabbit anti-human GPNMB polyclonal IgG antibody (diluted 1/100) and Alexa488 goat anti rabbit IgG secondary antibody. Nuclei were counterstained with DAPI. |
|
| Figure 2 | 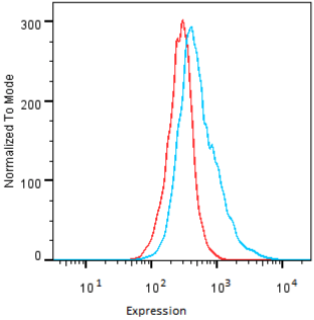 |
| Flow Cytometry. HeLa cells untransfected (red) and ICAFectin441-transfected with a plasmid encoding human GPNMB (blue) were stained with rabbit anti-human GPNMB polyclonal IgG antibody (diluted 1/100) and R-PE goat anti rabbit IgG secondary antibody. |
|
| Figure 3 | 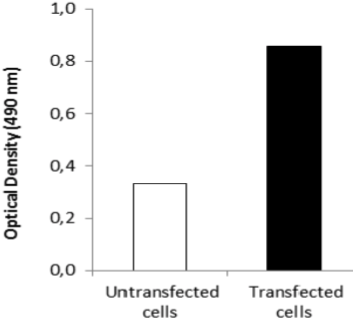 |
| ELISA. Lysates of HeLa cells untransfected (red) and ICAFectin441-transfected with a plasmid encoding human GPNMB were coated in 96 wells plate. Wells were stained with anti-human GPNMB polyclonal IgG and HRP goat anti rabbit IgG secondary antibody. |
|
| Product name | Anti Human/Rat/Mouse Transmembrane Glycoprotein NMB (GPNMB) pAb (Rabbit, Purified Ig) |
| Cat No | CAC-ICA-TG1-RBP3 |
| Description |
Originally described in melanoma cells, glycoprotein nonmetastatic melanoma B (Gpnmb), also known as osteoactivin and dendritic cell-associated heparin sulfate proteoglycan-dependent integrin ligand, is a heavily N-glycosylated type I transmembrane domain protein with a short cytoplasmic domain containing an endosomal sorting motif. Gpnmb is expressed in numerous cell types, including dendritic cells, macrophages, retinal pigment epithelial cells, osteoblasts and osteoclasts. Recent studies have demonstrated that Gpnmb expressed in macrophages functions as a feedback regulator of proinflammatory responses, and that binding of Gpnmb on antigen-presenting cells to syndecan-4 on activated T cells inhibits T-cell activation. Thus, Gpnmb expressed on antigen-presenting cells may negatively regulate inflammation. Additionally, Gpnmb is involved in osteoblast maturation, and is also associated with motor neuron survival. We previously isolated the gene encoding Gpnmb and demonstrated that it was differentially expressed in the livers of rats fed a choline-deficient, L-amino acid-defined diet. We also found that transgenic Gpnmb expression attenuates the development of hepatic fibrosis. Furthermore, a recent report has demonstrated that Gpnmb is expressed in macrophages infiltrating into injured liver tissues. These findings suggest a pathophysiologic role for Gpnmb in various disorders, including liver injury. However, the specific nature of Gpnmb involvement in liver injury remains unknown. In this study, we focused on the role of infiltrating hepatic macrophages, especially those expressing Gpnmb, during the repair process in injured livers in a mouse model of acute liver injury. [from: Kumagai K, Tabu K, Sasaki F, Takami Y, Morinaga Y, Mawatari S, et al. (2015) Glycoprotein Nonmetastatic Melanoma B (Gpnmb)-Positive Macrophages Contribute to the Balance between Fibrosis and Fibrolysis during the Repair of Acute Liver Injury in Mice. PLoS ONE 10(11): e0143413. https://doi.org/10.1371/journal.pone.0143413] Lipid-laden macrophages may orchestrate pathology, an accepted notion for inborn lysosomal storage disorders (LSDs) and more recently for metabolic syndrome. The development of enzyme replacement therapy (ERT) for specific LSDs has led in the last decades to the identification of markers of lipid-laden macrophages. In LSDs characterized by foamy macrophages as storage cells, plasma GPNMB has been shown to accurately reflect disease burden. Moreover, GPNMB is also applicable in mouse models of LSDs like Gaucher disease and Niemann-Pick type C. GPNMB is also increased in several acquired diseases, such as metabolic syndrome and neurodegeneration. It therefore might be that these disease conditions share pathophysiological elements, in particular the accumulation of foamy, lysosomal stressed, macrophages. GPNMB is among the most upregulated proteins in lipid-laden macrophages. Nevertheless, at present its exact function in foamy macrophage remains largely enigmatic. Important unanswered questions concern the function(s) served by GPNMB, either the cellular membrane-bound or (extracellular) soluble isoforms, in lipid-laden macrophages and beyond. |
| Host | Rabbit |
| Species specificity | HU MS RT |
| Figure 1 | 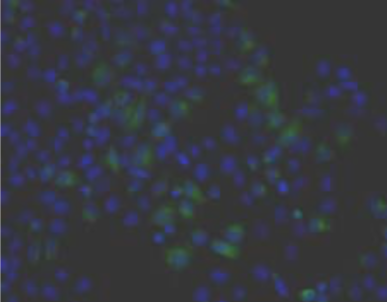 |
| Immunohistochemistry. Hela cells ICAFectin441-transfected with a plasmid encoding human GPNMB were fixed, permeabilized and stained with rabbit anti-human GPNMB polyclonal IgG antibody (diluted 1/100) and Alexa488 goat anti rabbit IgG secondary antibody. Nuclei were counterstained with DAPI. |
|
| Figure 2 | 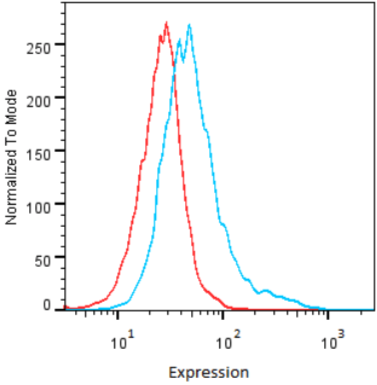 |
| Flow Cytometry. HeLa cells untransfected (red) and ICAFectin441-transfected with a plasmid encoding human GPNMB (blue) were stained with rabbit anti-human GPNMB polyclonal IgG antibody (diluted 1/100) and R-PE goat anti rabbit IgG secondary antibody. |
|
| Figure 3 | 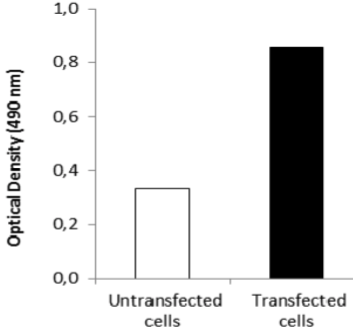 |
| ELISA. Lysates of HeLa cells untransfected (red) and ICAFectin441-transfected with a plasmid encoding human GPNMB were coated in 96 wells plate. Wells were stained with anti-human GPNMB polyclonal IgG and HRP goat anti rabbit IgG secondary antibody. |
|
| Inflammatory cytokines (Top) | |
| Human recombinant IL-32 does not exhibit similarities with known cytokine families, yet several properties are typical of a pro-inflammatory cytokine. It was discovered accidentally while studying the genes induced by IL-18 and was found to stimulate the production of various chemokines, pro-inflammatory cytokines including IL-1β, IL-6, IL-8, TNF-α and macrophage inflammatory protein-2 (MIP-2). Inflammation or infection with various pathogens including Mycobacterium tuberculosis, Epstein-Barr virus (EBV), human immunodeficiency virus (HIV) and influenza A virus have been reported to induce the expression of IL-32. The IL-32 gene is located on human chromosome 16 p13.3, which is organized into eight exons with six splice variants of the gene; these variants have been described as IL-32α, IL-32β, IL-32γ, IL-32δ, IL-32ε and IL-32ζ, of which, IL-32α is the most abundant transcript. Anti-tumor activity of NK cells is provoked by IL-12 and IL-18, both of which induce IL-32 production that stimulates TNF-α synthesis enhancing NK apoptotic activity. IL-32 was found in cytosol as well as in the nucleus. Park et al. reported that IL-32 enhances the anti-tumor activity specifically for NK-92 cells upon introduction of the death receptor and the activation of caspase-3 pathway in cancer cells. IL-32 has been reported to play a key role in the pathogenesis of various disorders, including infectious autoimmune and inflammatory diseases. | |
| Product name | Anti Interleukin-32 (IL-32) pAb (Rabbit, Antiserum) |
| Cat No | CAC-SU-IZ-P02 |
| Description | Anti-IL-32 polyclonal antibody obtained from rabbit immunization with purified E. coli-derived recombinant human IL-32. This antibody can be used for the detection of IL-32 by immunoblotting and flow cytometry. References: Goda, C et al., Int. Immunol. 18:233-240, 2006 |
| Host | Rabbit |
| Species specificity | HU |
| Figure 1 | 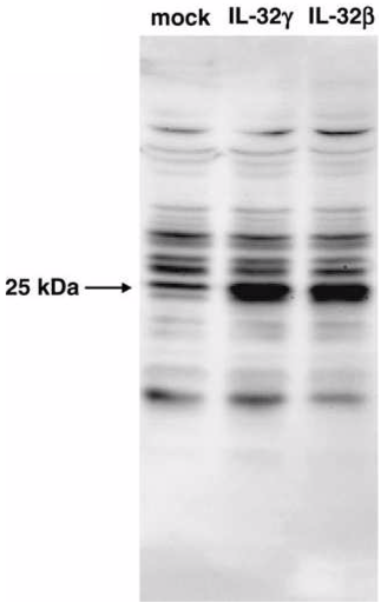 |
| Cell lysates prepared from mock- or IL-32(gamma)- or IL-32(beta)-transfected HEK293T cells were analyzed by western blot using anti-IL-32 serum. | |
| Viral recognition pathways (Top) | |
| RIG-I like receptors (RLRs) are a family of DExD/H box RNA helicases that function as cytoplasmic sensors of pathogen-associated molecular patterns (PAMPs) within viral RNA (reviewed in (Onoguchi, Yoneyama et al. 2011)). The RLRs signal downstream transcription factor activation to drive type 1 interferon (IFN) production and antiviral gene expression that elicits an intracellular immune response to control virus infection. To date, three RLR members have been identified: RIG-I (retinoic acid-inducible gene I) – the founding member and therefore best characterized of this family, MDA5 (melanoma differentiation associated factor 5), and LGP2 (laboratory of genetics and physiology 2 and a homolog of mouse D11lgp2). | |
| Product name | Anti Probable ATP-Dependent RNA Helicase DDX58 (RIG-I/RLR-1) mAb (Clone SS1A) |
| Cat No | CAC-SU-IZ-M02 |
| Description | Anti-RIG-I antibody is a rat monoclonal antibody obtained from immunization with a 16 amino acid peptide from the C-terminus of mouse RIG-I. This antibody can be used for the detection of mouse RIG-I by immunoblotting. |
| Host | Rat |
| Species specificity | MS |
| Vpr for HIV research (Top) | |
| Viral Protein R (Vpr), an accessory gene of human immunodeficiency virus type 1 (HIV-1), encodes a virion-associated nuclear protein with a variety of biological functions. Forced expression of Vpr induces abnormalities in the cell cycle at the G2/M phase, macrophage infection, apoptosis and nuclear transportation of the pre-integration complex. Vpr is a transacting factor and exogenously added Vpr can also induce apoptosis. It has been reported that Vpr is present in blood and cerebrospinal fluids of HIV-1 positive patients. Vpr may also be linked with functional abnormality of the central nervous system. Recently, it was reported that Vpr induces DNA double-strand breaks (DSBs) and activates ATM and H2AX. ATM is considered a major physiological mediator of H2AX mobilization in response to DSB formation. Foci of ATM phosphorylated at serine 1981 (ATM-p) and H2AX were observed in cells with Vpr expression. | |
| Product name | Anti HIV-1 Viral protein R (Vpr) mAb (Clone 8D1) |
| Cat No | CAC-NCG-M01 |
| Description | Feature and advantages of Clone 8D1: • Vpr is detected in the blood of about 40% of HIV-positive individuals. • Vpr in the blood of HIV-positive individuals is detected in the nM-pM range. • Useful in western blotting, FACS, immunoprecipitation and also in neutralizing activity. • High-purity recombinant Vpr (rVpr) can be purified by affinity chromatography. References: 1) Daiki Taneichi, et al, Identification of SNF2h, a Chromatin-Remodeling Factor, as a Novel Binding Protein of Vpr of Human Immunodeficiency Virus Type 1 J Neuroimmune Pharmacol (2011) 6:177.187. PMID: 21519849 2) Mari Shimura, et al, Epigenetic displacement of HP1 from heterochromatin by HIV-1 Vpr causes premature sister chromatid separation J Cell Biol. 2011 Sep 5; 194(5):721-35. Epub 2011 Aug 29. PMID: 21875947 3) Tram N. Q. Pham, et al, Modulation of NKG2D-Mediated Cytotoxic Functions of Natural Killer Cells by Viral Protein R from HIV-1 Primary Isolates: J.Virol. 85: 12254-12261 (2011). PMID: 21957298 4) Shigeki Hoshino, et al, HIV-1 Vpr induces TLR4/MyD88-mediated IL-6 production and reactivates viral production from latency. J. Leukoc Biol. 87:1133-1143 (2010). PMID: 20145198 5) Hoshino, S., et al, Vpr in plasma of HIV-1-positive patients is correlated with the HIV-1 RNA titers. AIDS Res. Hum. Retrovir. 23, 391-397 (2007). PMID: 17411372 6) Nakai-Murakami C, et al, HIV-1 Vpr induces ATM-dependent cellular signal with enhanced homologous recombination. Oncogene. 2007 Jan 25;26(4):477-86. PMID: 16983346 |
| Host | Mouse |
| Species specificity | HIV-1 |
| Figure 1 | 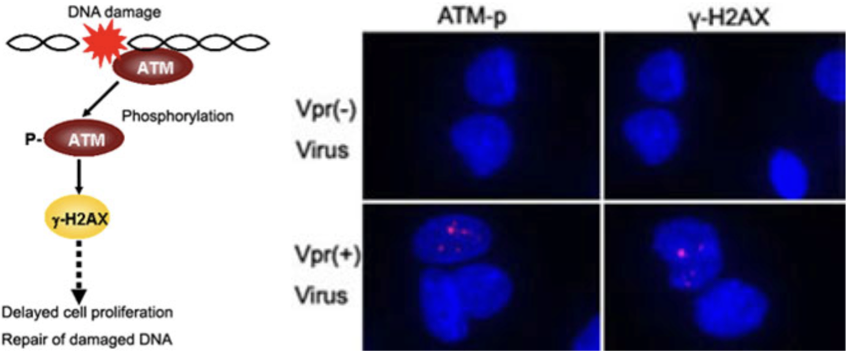 |
| Focus formation of phosphorylation of ATM (ATM-p) and γ-H2AX following Vpr expression in MIT-23 cells. Cells were stained with antibodies against ATM-p and γ-H2AX. Signals for ATM-p and γ-H2AX are depicted as red spots in the nucleus (blue). |
|
| Figure 2 | 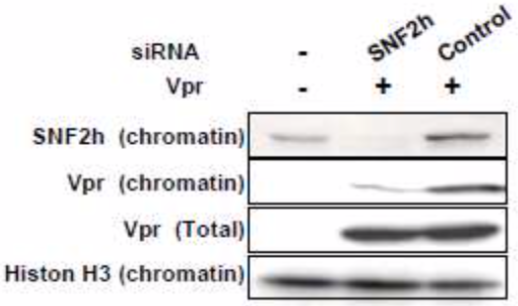 |
| Western blot with 8D1 antibody using whole cell extracts of chromatin fraction. Chromatin fraction was prepared from HEK293T cells transfected with pCMV-Vpr and either control or SNF2h siRNAs (see Ref. 1 Fig. 3). lane 1: vector control lane 2: pCMV-Vpr with SNF2h siRNA lane 3: pCMV-Vpr with control siRNA |
|
| Figure 3 | 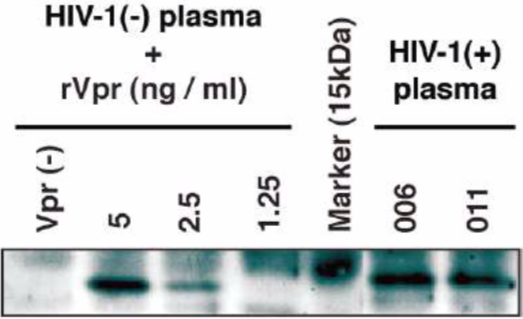 |
| IP-WB analysis of Vpr in plasma of HIV-1-positive patients. A definite signal of the 14-kDa protein was observed in patients #006 and #011 (see Ref. 5 Fig. 1). lane1: rVpr(-) lane 2: 5 ng of standard rVpr lane 3: 2.5 ng of standard rVpr lane 4: 1.25 ng of standard rVpr lane 5: Molecular marker lane 6: HIV-1-positive plasma #006 lane 7: HIV-1-positive plasma #011 |
|
| Figure 4 | 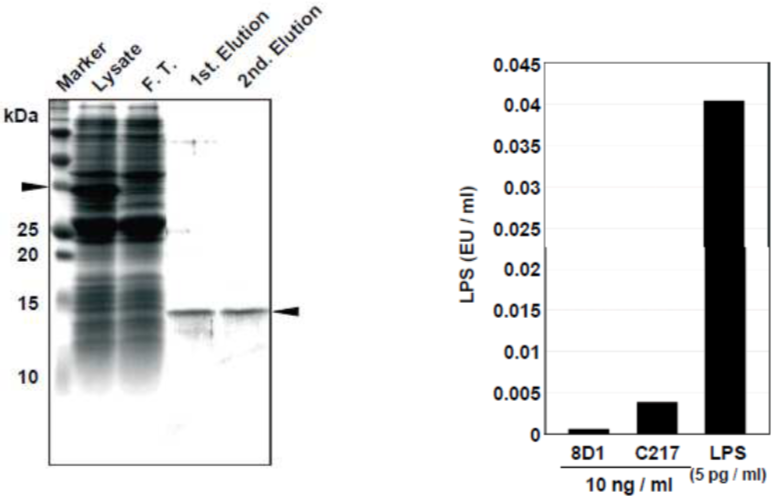 |
| LPS-free Vpr protein purification using a glutathione column and an affinity column with anti-Vpr antibody (8D1) (see Ref. 4 Fig. 1). | |
