- CAC Antibody Collection Index Page
Antibody Group
Advanced glycation end products (AGEs)
Autophagy and apoptosis
Bacteria-related
Calcium-binding proteins
Cancer
CD44 for enriching cancer stem cells
Tumor markers
Tumor inhibitors
Chaperones
Cytoskeleton
DNA damage
UV-induced DNA lesions
8-Nitroguanosine for oxidative stress research
Nucleotide excision repair factors
Epigenetics and chromatin
Histone H3 variants
Post-translationally-modified histone H3
Chromatin structure modifiers
Drosophila chromatin
Epitope tags
Exosomes
Extracellular matrix
Glycosaminoglycans (GAGs)
Proteoglycans
Matrix and basement membrane
Cell adhesion and hemidesmosome-related
Bone and cartilage-related
Wound repair-related
Hedgehog pathway
Hormones
Immunology
Fish CD4 and CD8α
Allergic disease-related
Adaptive and innate immunity
Macrophages
Inflammatory cytokines
Viral recognition pathways
Vpr for HIV research
Insulin-like growth factor-related
Mitochondria-related
Neurobiology
Neurodegenerative disease markers
Muscarinic acetylcholine receptors
Miscellaneous
Nuclear import and export
Oxidative stress
Plant-related
Plant hormones
Plant autophagy and apoptosis
Plant stress response
Plant stress response
Proteasomes
Puromycin-specific
Reproductive biology
Small molecules
Stem cells
Novel iPS/ES markers
Pluripotency-associated
Sumoylation pathway
TGF-beta pathway
TGF-beta LAP-d
TGF-beta signaling
Transcription factors
Transporters
Tyrosine phosphatases
Ubiquitin-Proteasome Related
 CAC Antibody Collection
CAC Antibody Collection
The antibodies on this page are part of Cosmo Bio's exclusive CAC Collection. For many many thousands of other antibodies from many different makers, use our Search the Store function and our Explore Products drop down menu.
CD44 for enriching cancer stem cells / Tumor markers / Tumor inhibitors / Tumor promotors
| Cancer: CD44 splice variants for cancer stem cell enrichment | |||
| Product name (click for order info) | Cat No (click for datasheet) |
Host | Species specificity |
| Anti CD44 Antigen v9 mAb (Clone RV3) | CAC-LKG-M003 | Rat | HU |
| Anti CD44 Antigen v10-e16 mAb (Clone RM1) | CAC-LKG-M002S | Rat | MS |
| Cancer: Tumor inhibitors | |||
| Product name (click for order info) | Cat No (click for datasheet) |
Host | Species specificity |
| Anti Prohibitin-1 (PHB1) mAb (Clone 1G5C1) | CAC-CE-051 | RT | HU MS RT MKY |
| Anti Prohibitin-2 (PHB2) mAb (Clone 7F8B3) | CAC-CE-052 | RT | HU MS RT MKY |
| Cancer: Tumor promotors | |||
| Product name (click for order info) | Cat No (click for datasheet) |
Host | Species specificity |
| Anti Proprotein Convertase Subtilisin/Kexin Type 6 (PACE4) - HomoB domain pAb (Rabbit, Antiserum) | CAC-SK-T01-001 | RAB | HU |
| Anti Proprotein Convertase Subtilisin/Kexin Type 6 (PACE4) - Propeptide pAb (Rabbit, Antiserum) | CAC-SK-T01-002 | RAB | HU |
CD44 for enriching cancer stem cells
CD44 is a single-pass type I transmembrane protein and functions as a cellular adhesion molecule for hyaluronic acid, a major component of the extracellular matrix. It exists in numerous isoforms that are generated through alternative splicing of CD44 precursor mRNA. Whereas the standard isoform of CD44 (CD44s) is expressed predominantly in hematopoietic cells and normal epithelial cell subsets, CD44v (variant) isoforms, which contain additional insertions in the membrane-proximal extracellular region, are highly expressed in epithelial-type carcinomas. Moreover, CD44 is reported to be a cell surface marker for cancer stem cells (CSCs) derived from solid tumors including breast, prostate, colon, head and neck and pancreatic cancer. Expression of CD44, especially variant isoforms (CD44v8-10), contributes to reactive oxygen species (ROS) defense through upregulation of the synthesis of reduced glutathione (GSH), the primary intracellular antioxidant. CD44v8-10 interacts with and stabilizes xCT, a subunit of the cystine-glutamate transporter xc(-), and thereby promotes cystine uptake for GSH synthesis. The ability to avoid the consequences of exposure to high levels of ROS is required for cancer cell survival and propagation in vivo. CSCs (whose defense against ROS is enhanced by CD44v8-10) are thus thought to drive tumor growth, chemoresistance and metastasis. Both clone RV3 (a monoclonal antibody specific for human CD44v9) and clone RM1 (a monoclonal antibody specific for mouse CD44v10-e16) can be used in flow cytometry, and importantly, for the enrichment of CSCs using FACS. They can be applied towards understanding a variety of molecular mechanisms and towards the development of new medicines against cancer stem cells using in vitro cell-based assays such as the "in vitro sphere formation" and "in vivo lung metastasis" assays. Click HERE for an annotated bibliography of these antibodies.
| Product name | Anti CD44 Antigen v9 mAb (Clone RV3) |
| Cat No | CAC-LKG-M003 |
| Description | Clone RV3 (a monoclonal antibody specific for human CD44 v9) can be used in flow cytometry, and importantly, for the enrichment of CSCs using FACS. RV3 can be applied towards understanding a variety of molecular mechanisms and towards the development of new medicines against cancer stem cells using in vitro cell-based assays such as the "in vitro sphere formation" and "in vivo lung metastasis" assays References: 1) Nagano O., et al., Oncogene. 2013 Jan 21., 1-8. PMID:23334333 2) Ishimoto T., et al., Cancer Cell. 2011 Mar 8;19(3):387-400. PMID : 21397861 3) Yae T., et al., Nat Commun. 2012 Jun 6;3:883. PMID: 22673910 4) Tsugawa H., et al., Cell Host Microbe. 2012 Dec 13;12(6):764-77. PMID: 23245321 5) Tanabe KK., et al., Lancet. 1993 Mar 20;341(8847):725-6. PMID: 8095628 |
| Host | Rat |
| Species specificity | HU |
| Figure 1 | 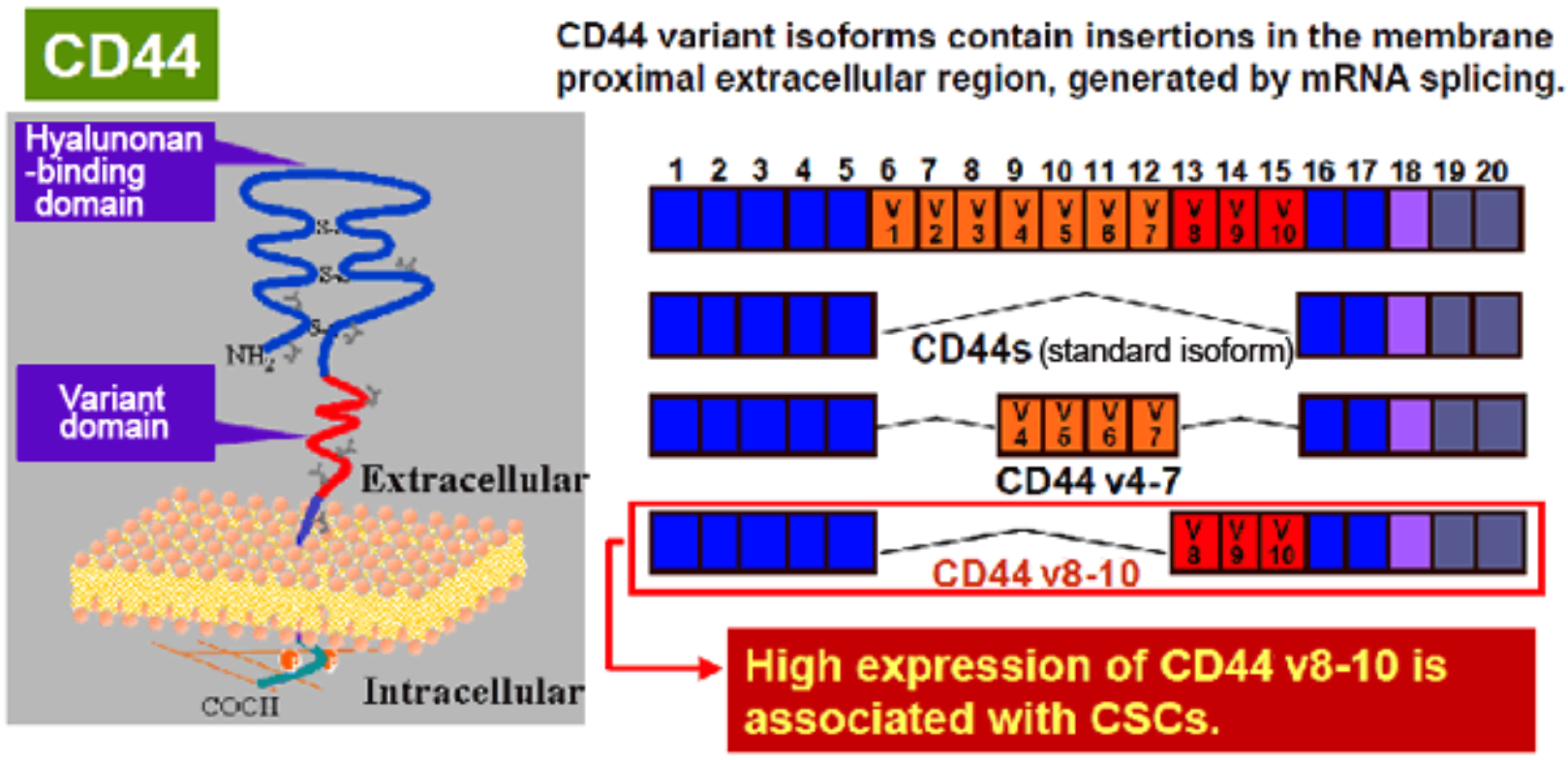 |
| CD44 is a single-pass type I transmembrane protein that functions as a cellular adhesion molecule for hyaluronic acid, a major component of the extracellular matrix. It exists in numerous isoforms that are generated through alternative splicing of CD44 precursor mRNA. | |
| Figure 2 | 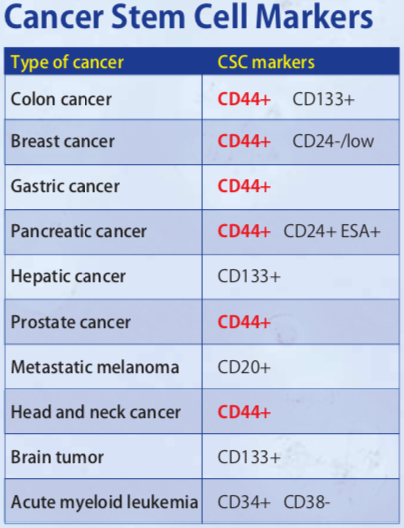 |
| CD44 is a cancer stem cell marker. | |
| Figure 3 | 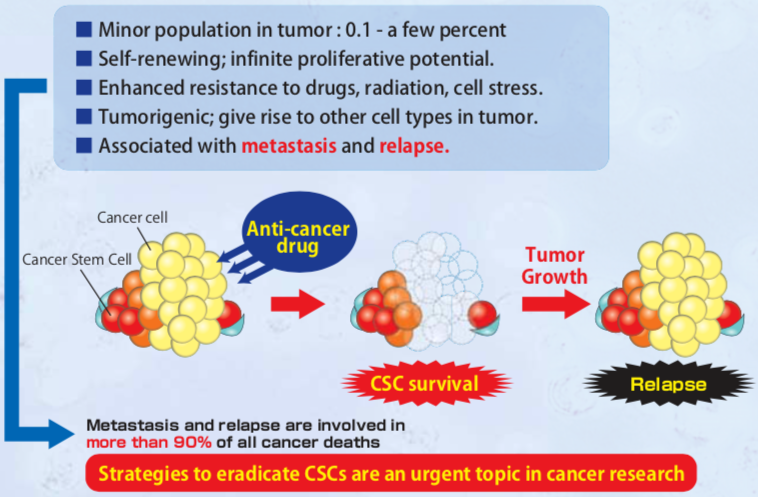 |
| Cancer stem cell characteristics. | |
| Figure 4 | 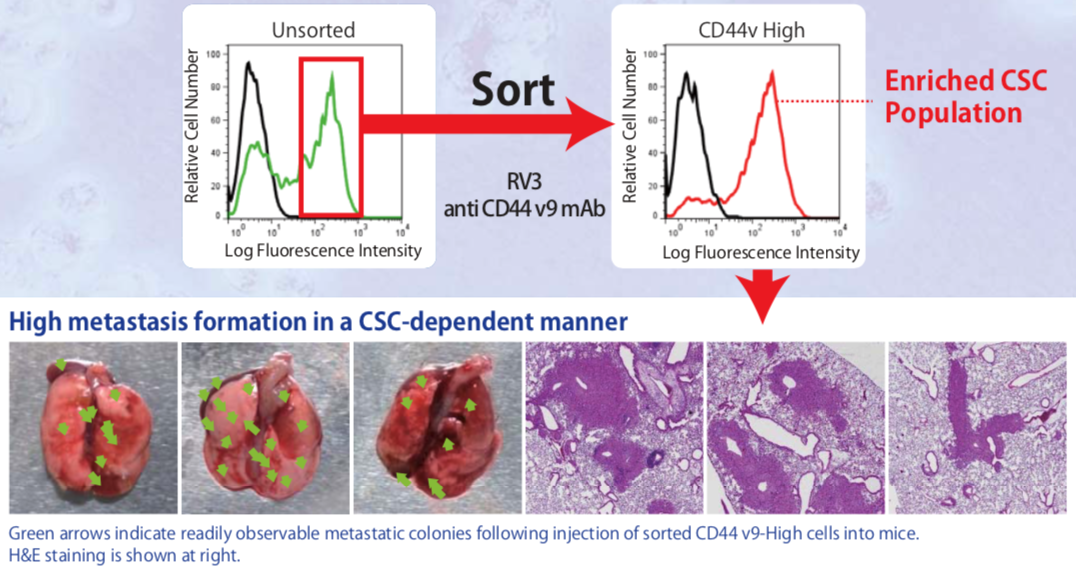 |
| In vivo lung metastasis assay showing the high lung colonization efficiency of CD44v9-high cell populations sorted from the human pancreatic cancer cell line AsPC-1. The high efficiency of metastasis (colony formation) by CD44v9-high cells presents an opportunity to assay the effectiveness of new anti-CSC therapeutic strategies. | |
| Figure 5 | 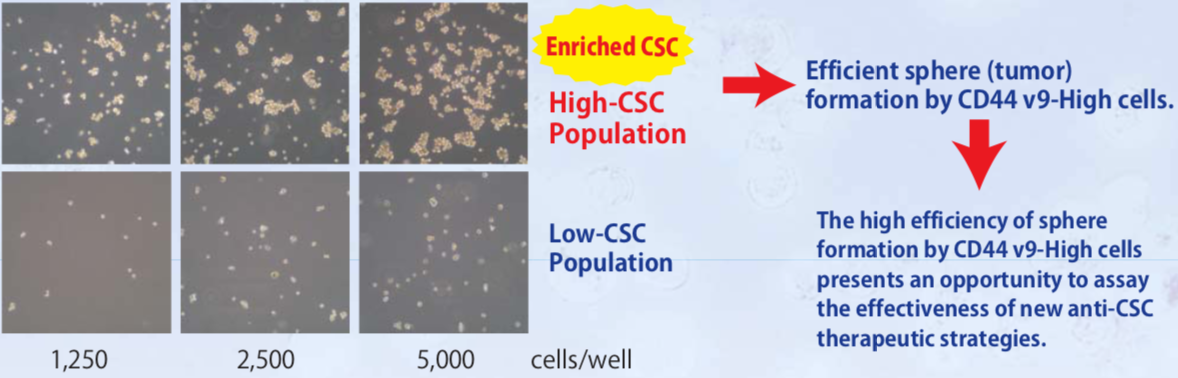 |
| In vitro Sphere formation assays with CD44v9-sorted human PC3 prostate cancer cells. | |
| Figure 6 | 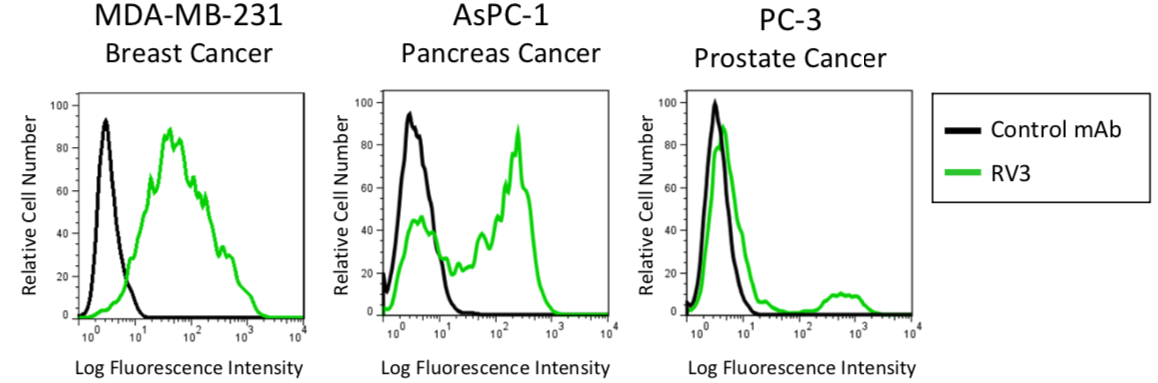 |
| Flow cytometric analysis of CD44v in human cancer cell lines using anti-CD44v9 (RV3, 3μg/mL) antibody and PE-labeled anti Rat IgG antibody. | |
| Figure 7 | 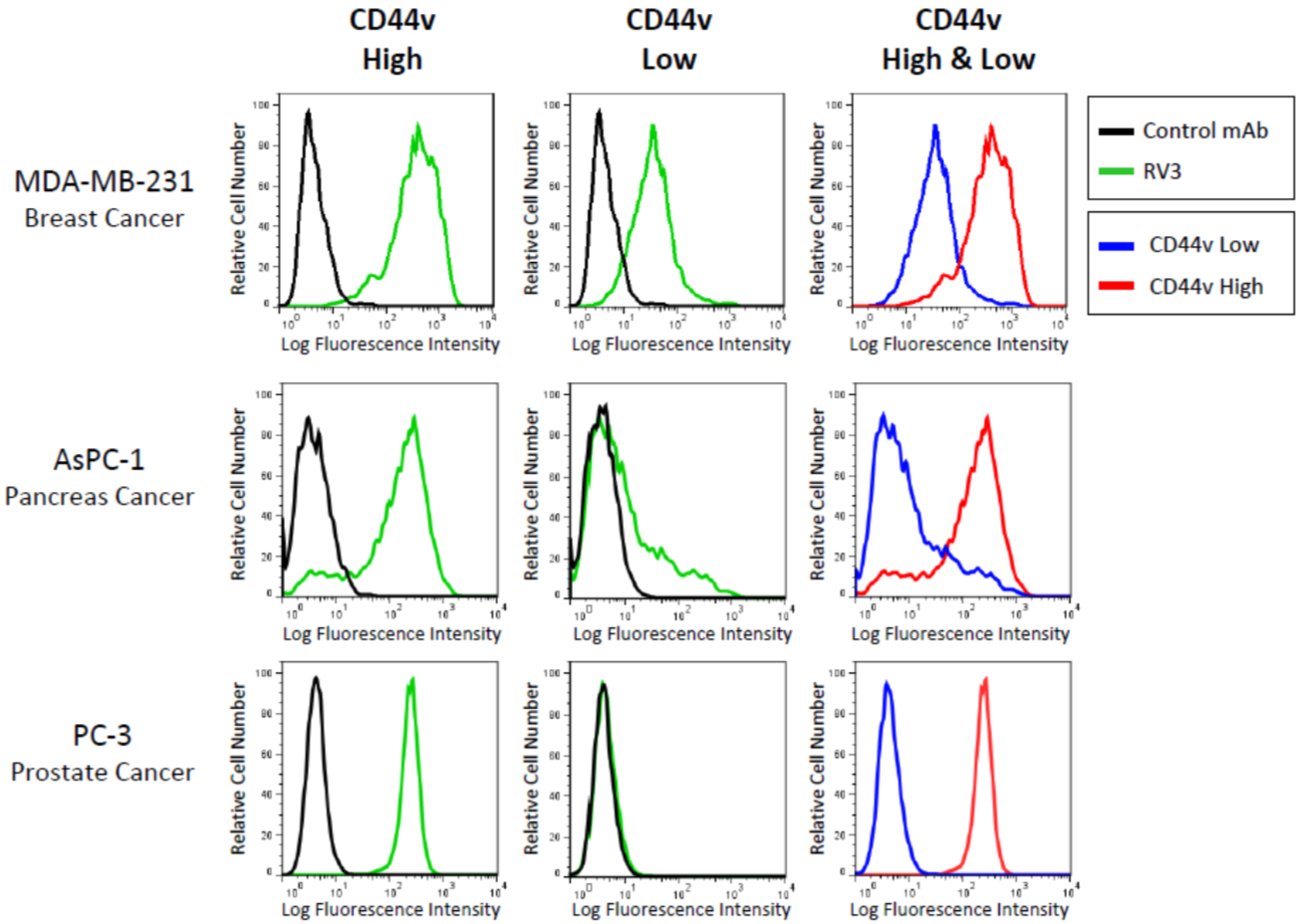 |
| Flow cytometric cell sorting on CD44v expression level in human cancer cell lines using anti-CD44v9 (RV3) antibody and PE-labeled anti Rat IgG antibody. Two kinds of subpopulations were isolated: CD44v+(High) and CD44v-(Low). | |
| Figure 8 |  |
| Immunohistochemical staining of invasive breast ductal carcinoma with anti-CD44v9 antibody (clone RV3, 0.2μg/mL). | |
| Figure 9 | 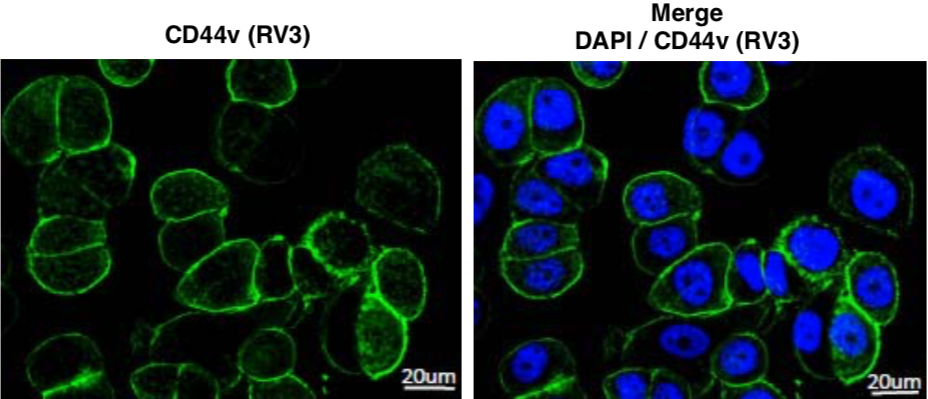 |
| Immunofluorescence staining of CD44v (green) in MDA-MB-468 cells using anti-CD44v9 antibody (RV3, 3μg/mL). | |
| Figure 10 | 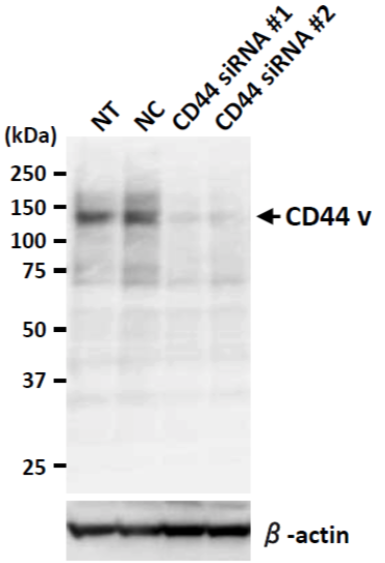 |
| Western blot analysis of CD44v in BT20 cell lysates using anti-CD44v9 antibody (RV3, 1μg/mL). NT: no treated NC: non-targeting siRNA-treated sample. | |
| Product name | Anti CD44 Antigen v10-e16 mAb (Clone RM1) |
| Cat No | CAC-LKG-M002 |
| Description | Clone RM1 (a monoclonal antibody specific for mouse CD44 v10-e16) can be used in flow cytometry, and importantly, for the enrichment of CSCs using FACS. RM1 can be applied towards understanding a variety of molecular mechanisms for cancer stem cells using in vitro cell-based assays such as “in vitro sphere formation” and “in vivo lung metastasis" assays. References: Nagano O., et al., Oncogene. 2013 Jan 21., 1-8. PMID:23334333 2) Ishimoto T., et al., Cancer Cell. 2011 Mar 8;19(3):387-400. PMID : 21397861 3) Yae T., et al., Nat Commun. 2012 Jun 6;3:883. PMID: 22673910 4) Tsugawa H., et al., Cell Host Microbe. 2012 Dec 13;12(6):764-77. PMID: 23245321 5) Tanabe KK., et al., Lancet. 1993 Mar 20;341(8847):725-6. PMID: 8095628 |
| Host | Rat |
| Species specificity | MS |
| Figure 1 | 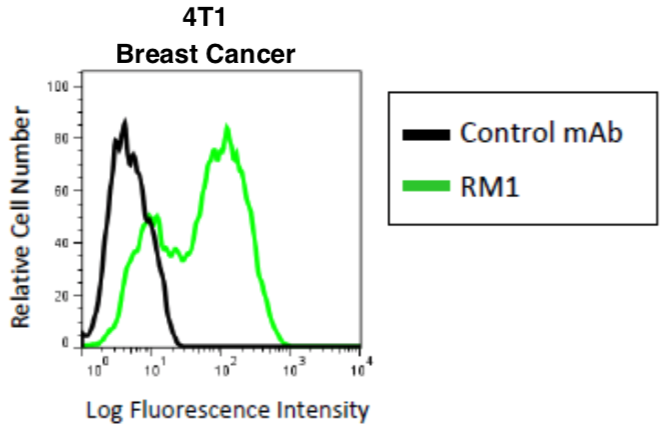 |
| Flow cytometric analysis of CD44v in mouse breast cancer cell line 4T1 with using-CD44v10-e16 (RM1, 3μg/mL) antibody and PE-labeled anti Rat IgG antibody. | |
| Figure 2 | 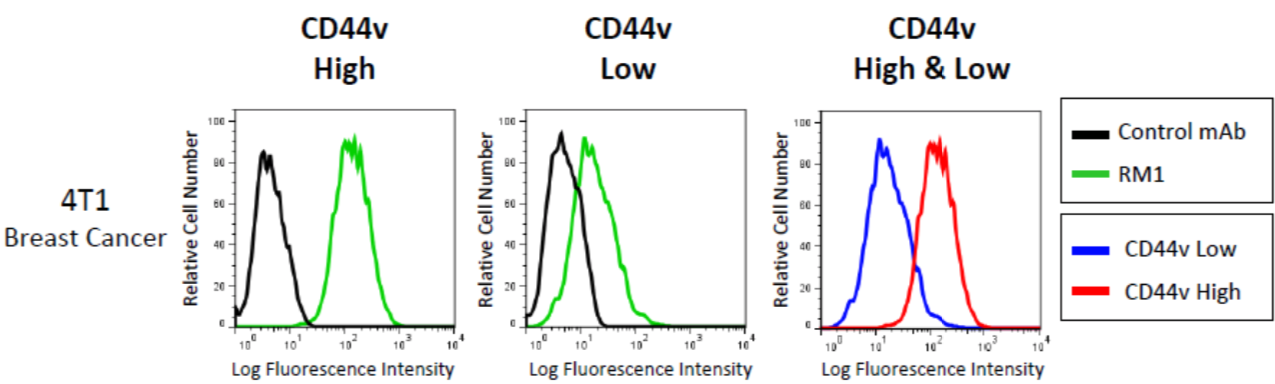 |
| Flow cytometric cell sorting on CD44v expression level in mouse breast cancer cell line 4T1 using anti-CD44v10-e16 (RM1) antibody and PE-labeled anti Rat IgG antibody. Two kinds of subpopulations were isolated: CD44v+(High) and CD44v-(Low). | |
| Tumor markers |
|
| Tumor markers are substances that are produced by cancer or by other cells of the body in response to cancer or certain benign (noncancerous) conditions. Most tumor markers are made by normal cells as well as by cancer cells; however, they are produced at much higher levels in cancerous conditions. These substances can be found in the blood, urine, stool, tumor tissue, or other tissues or bodily fluids of some patients with cancer. Most tumor markers are proteins. However, more recently, patterns of gene expression and changes to DNA have also begun to be used as tumor markers. Many different tumor markers have been characterized and are in clinical use. Some are associated with only one type of cancer, whereas others are associated with two or more cancer types. No “universal” tumor marker that can detect any type of cancer has been found. There are some limitations to the use of tumor markers. Sometimes, noncancerous conditions can cause the levels of certain tumor markers to increase. In addition, not everyone with a particular type of cancer will have a higher level of a tumor marker associated with that cancer. Moreover, tumor markers have not been identified for every type of cancer. Because tumor markers can be used to assess the response of a tumor to treatment and for prognosis, researchers have hoped that they might also be useful in screening tests that aim to detect cancer early, before there are any symptoms. For a screening test to be useful, it should have very high sensitivity (ability to correctly identify people who have the disease) and specificity (ability to correctly identify people who do not have the disease). If a test is highly sensitive, it will identify most people with the disease—that is, it will result in very few false-negative results. If a test is highly specific, only a small number of people will test positive for the disease who do not have it—in other words, it will result in very few false-positive results. Although tumor markers are extremely useful in determining whether a tumor is responding to treatment or assessing whether it has recurred, no tumor marker identified to date is sufficiently sensitive or specific to be used on its own to screen for cancer. For example, the prostate-specific antigen (PSA) test, which measures the level of PSA in the blood, is often used to screen men for prostate cancer. However, an increased PSA level can be caused by benign prostate conditions as well as by prostate cancer, and most men with an elevated PSA level do not have prostate cancer. Initial results from two large randomized controlled trials, the NCI-sponsored Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO), and the European Randomized Study of Screening for Prostate Cancer, showed that PSA testing at best leads to only a small reduction in the number of prostate cancer deaths. Moreover, it is not clear whether the benefits of PSA screening outweigh the harms of follow-up diagnostic tests and treatments for cancers that in many cases would never have threatened a man’s life. [from: NIH NCI https://www.cancer.gov/about-cancer/diagnosis-staging/diagnosis/tumor-markers-fact-sheet] |
|
| Product name | Anti DEP Domain-Containing Protein 1B (XTP1/XTP8) mAb (Clone 2191H11) |
| Cat No | CAC-PRPG-XTP-M01 |
| Description | The RhoGAP family embraces a unique member named XTP1 (also referred to as DEPDC1B, BRCC3 or FLJ11252) that pairs with a homologue denoted SDP35 (also referred to as DEPDC1, DEP8, FLJ20354 or DEPDC1-V2). The structural-functional properties of XTP1 are still largely unknown, but its structural uniqueness resides in the presence of a domain showing homology with Dishevelled, i.e. the DEP domain (Dishevelled/Pleckstrin-like domain). The presence of this domain suggests that XTP1 might engage in more complex molecular interactions than those of other members of the family. Another peculiar feature of XTP1 is represented by its atypical GAP domain, which lacks the orthodox “Arg finger” catalytic motif essential for exerting canonical GAP function. Whereas most RhoGAP family members are either ubiquitously expressed throughout the body or are concentrated in discrete tissue/organs, XTP1 is remarkably poorly represented in most human adult tissues (also supported by evidence provided by the Comparative Cancer Genome Project database). XTP1 is de novo expressed upon neoplastic transformation and remains abundant in many cancer cell lines. Some observations in epithelial tumors suggest that it may act as a cell-cycle regulator. |
| Host | Mouse |
| Species specificity | HU |
| Product name | Anti DEP Domain-containing Protein 1A (SDP35) mAb (Clone 220D12) |
| Cat No | CAC-PRPG-SDP-M01 |
| Description | The RhoGAP family encompasses a unique member named SDP35 (also referred to as DEPDC1, DEP8, FLJ20354 or DEPDC1-V2) that pairs with a homologue named XTP1 (also referred to as DEPDC1B, BRCC3 or FLJ11252).The structural-functional properties of SDP35 are still largely unknown, but its structural uniqueness resides in the presence of a domain showing homology with Dishevelled, i.e. the DEP domain (Dishevelled/Pleckstrin-like domain). The presence of this domain suggests that SDP35 might engage in more complex molecular interactions than those of other members of the family. Another peculiar feature of SDP35 is represented by its atypical GAP domain, which lacks the orthodox "Arg finger" catalytic motif essential for exerting canonical GAP function. Whereas most RhoGAP family members are either ubiquitously expressed throughout the body or are concentrated in discrete tissue/organs, SDP35 is remarkably poorly represented in most human tissues (also supported by evidence provided by the Comparative Cancer Genome Project database). SDP35 has been reported to be up-regulated in bladder cancer and numerous cancer cell types. |
| Host | Mouse |
| Species specificity | HU |
| Product name | Anti Serpin B3 (SCCA1/T4-A) mAb (Clone SS6C) |
| Cat No | CAC-SU-IZ-M08 |
| Description | Squamous cell carcinoma antigen (SCCA) is a member of the ovalbumin family of serine proteinase inhibitors. The protein was isolated from a metastatic cervical squamous cell carcinoma by Kato and Torigoe (1977). SCCA is detected in the superficial and intermediate layers of normal squamous epithelium, whereas the mRNA is detected in the basal and sub-basal levels. The clinical import of SCCA has been as a circulating tumor marker for squamous cell carcinoma, especially those of the cervix, head and neck, lung, and esophagus. Many clinical studies of cervical squamous cell carcinoma show that the percentage of patients with elevated circulating levels of SCCA increases from approximately 12% at stage 0 to more than 90% at stage IV. Levels fall after tumor resection and rise in approximately 90% of the patients with recurrent disease. Similar trends occur in the other types of squamous cell carcinoma, with a maximum sensitivity of approximately 60% for lung, 50% for esophageal, and 55% for head and neck tumors. The neutral form of SCCA (SCCA1, or SERPINB3) is detected in the cytoplasm of normal and some malignant squamous cells, whereas the acidic form (SCCA2, or SERPINB4) is expressed primarily in malignant cells and is the major form found in the plasma of cancer patients. Thus, the appearance of the acidic fraction of SCCA is correlated with more aggressive tumors (summary by Schneider et al., 1995). Antibody source: Professor Kenji Dehara, Professor of Molecular Life Science, Faculty of Medicine, Saga University. References: 1) The usefulness of combined measurements of squamous cell carcinoma antigens 1 and 2 in diagnosing atopic dermatitis. Shoichiro Ohta, et al. 2012. Ann Clin Biochem. 49: 277-284. 2) Characterization of novel squamous cell carcinoma antigen-related molecules in mice. Y. Sakata, et al. 2004. Biochem Biophys Res Commun. 324(4):1340-1345. 3) The squamous cell carcinoma antigens as relevant biomarkers of atopic dermatitis. K. Mitsuishi, et al. 2005. Clin Exp Allergy 35:1327-1333. 4) Involvement of IL-32 in activation-induced cell death in T cells. Chiho Goda, et al. 2006. Int Immunol 18(2):233-240. |
| Host | Rat |
| Species specificity | HU |
| Product name | Anti Serpin B4 (Leupin/SCCA2) mAb (Clone SS8J) |
| Cat No | CAC-SU-IZ-M01 |
| Description | Squamous cell carcinoma antigen (SCCA) is a member of the ovalbumin family of serine proteinase inhibitors. The protein was isolated from a metastatic cervical squamous cell carcinoma by Kato and Torigoe (1977). SCCA is detected in the superficial and intermediate layers of normal squamous epithelium, whereas the mRNA is detected in the basal and sub-basal levels. The clinical import of SCCA has been as a circulating tumor marker for squamous cell carcinoma, especially those of the cervix, head and neck, lung, and esophagus. Many clinical studies of cervical squamous cell carcinoma show that the percentage of patients with elevated circulating levels of SCCA increases from approximately 12% at stage 0 to more than 90% at stage IV. Levels fall after tumor resection and rise in approximately 90% of the patients with recurrent disease. Similar trends occur in the other types of squamous cell carcinoma, with a maximum sensitivity of approximately 60% for lung, 50% for esophageal, and 55% for head and neck tumors. The neutral form of SCCA (SCCA1, or SERPINB3) is detected in the cytoplasm of normal and some malignant squamous cells, whereas the acidic form (SCCA2, or SERPINB4) is expressed primarily in malignant cells and is the major form found in the plasma of cancer patients. Thus, the appearance of the acidic fraction of SCCA is correlated with more aggressive tumors (summary by Schneider et al., 1995). Antibody source: Professor Kenji Dehara, Professor of Molecular Life Science, Faculty of Medicine, Saga University. References: 1) The usefulness of combined measurements of squamous cell carcinoma antigens 1 and 2 in diagnosing atopic dermatitis. Shoichiro Ohta, et al. 2012. Ann Clin Biochem. 49: 277-284. 2) Characterization of novel squamous cell carcinoma antigen-related molecules in mice. Y. Sakata, et al. 2004. Biochem Biophys Res Commun. 324(4):1340-1345. 3) The squamous cell carcinoma antigens as relevant biomarkers of atopic dermatitis. K. Mitsuishi, et al. 2005. Clin Exp Allergy 35:1327-1333. 4) Involvement of IL-32 in activation-induced cell death in T cells. Chiho Goda, et al. 2006. Int Immunol 18(2):233-240. |
| Host | Rat |
| Species specificity | HU |
| Product name | Anti Serpin B3 (SCCA1/T4-A) and Serpin B4 (SCCA2/Leupin) mAb (Clone SS6A) |
| Cat No | CAC-SU-IZ-M07 |
| Description | Squamous cell carcinoma antigen (SCCA) is a member of the ovalbumin family of serine proteinase inhibitors. The protein was isolated from a metastatic cervical squamous cell carcinoma by Kato and Torigoe (1977). SCCA is detected in the superficial and intermediate layers of normal squamous epithelium, whereas the mRNA is detected in the basal and sub-basal levels. The clinical import of SCCA has been as a circulating tumor marker for squamous cell carcinoma, especially those of the cervix, head and neck, lung, and esophagus. Many clinical studies of cervical squamous cell carcinoma show that the percentage of patients with elevated circulating levels of SCCA increases from approximately 12% at stage 0 to more than 90% at stage IV. Levels fall after tumor resection and rise in approximately 90% of the patients with recurrent disease. Similar trends occur in the other types of squamous cell carcinoma, with a maximum sensitivity of approximately 60% for lung, 50% for esophageal, and 55% for head and neck tumors. The neutral form of SCCA (SCCA1, or SERPINB3) is detected in the cytoplasm of normal and some malignant squamous cells, whereas the acidic form (SCCA2, or SERPINB4) is expressed primarily in malignant cells and is the major form found in the plasma of cancer patients. Thus, the appearance of the acidic fraction of SCCA is correlated with more aggressive tumors (summary by Schneider et al., 1995). Anti-SCCA1/2 antibody is a rat monoclonal antibody which obtained from the immunization with purified, E. coli-derived, recombinant human SCCA1. This antibody can be used for the detection of human SCCA1 and SCCA2 by immunoprecipitation and ELISA. References: 1) The usefulness of combined measurements of squamous cell carcinoma antigens 1 and 2 in diagnosing atopic dermatitis. Shoichiro Ohta, et al. 2012. Ann Clin Biochem. 49: 277-284. 2) Characterization of novel squamous cell carcinoma antigen-related molecules in mice. Y. Sakata, et al. 2004. Biochem Biophys Res Commun. 324(4):1340-1345. 3) The squamous cell carcinoma antigens as relevant biomarkers of atopic dermatitis. K. Mitsuishi, et al. 2005. Clin Exp Allergy 35:1327-1333. 4) Involvement of IL-32 in activation-induced cell death in T cells. Chiho Goda, et al. 2006. Int Immunol 18(2):233-240. |
| Host | Rat |
| Species specificity | HU |
| Product name | Anti Serpin B3 (SCCA1/T4-A) and Serpin B4 (SCCA2/Leupin) pAb (Rabbit, Antiserum) |
| Cat No | CAC-SU-IZ-P04 |
| Description | Squamous cell carcinoma antigen (SCCA) is a member of the ovalbumin family of serine proteinase inhibitors. The protein was isolated from a metastatic cervical squamous cell carcinoma by Kato and Torigoe (1977). SCCA is detected in the superficial and intermediate layers of normal squamous epithelium, whereas the mRNA is detected in the basal and sub-basal levels. The clinical import of SCCA has been as a circulating tumor marker for squamous cell carcinoma, especially those of the cervix, head and neck, lung, and esophagus. Many clinical studies of cervical squamous cell carcinoma show that the percentage of patients with elevated circulating levels of SCCA increases from approximately 12% at stage 0 to more than 90% at stage IV. Levels fall after tumor resection and rise in approximately 90% of the patients with recurrent disease. Similar trends occur in the other types of squamous cell carcinoma, with a maximum sensitivity of approximately 60% for lung, 50% for esophageal, and 55% for head and neck tumors. The neutral form of SCCA (SCCA1, or SERPINB3) is detected in the cytoplasm of normal and some malignant squamous cells, whereas the acidic form (SCCA2, or SERPINB4) is expressed primarily in malignant cells and is the major form found in the plasma of cancer patients. Thus, the appearance of the acidic fraction of SCCA is correlated with more aggressive tumors (summary by Schneider et al., 1995). References: 1) The usefulness of combined measurements of squamous cell carcinoma antigens 1 and 2 in diagnosing atopic dermatitis. Shoichiro Ohta, et al. 2012. Ann Clin Biochem. 49: 277-284. 2) Characterization of novel squamous cell carcinoma antigen-related molecules in mice. Y. Sakata, et al. 2004. Biochem Biophys Res Commun. 324(4):1340-1345. 3) The squamous cell carcinoma antigens as relevant biomarkers of atopic dermatitis. K. Mitsuishi, et al. 2005. Clin Exp Allergy 35:1327-1333. 4) Involvement of IL-32 in activation-induced cell death in T cells. Chiho Goda, et al. 2006. Int Immunol 18(2):233-240. |
| Host | Rabbit |
| Species specificity | HU |
| Figure 1 | 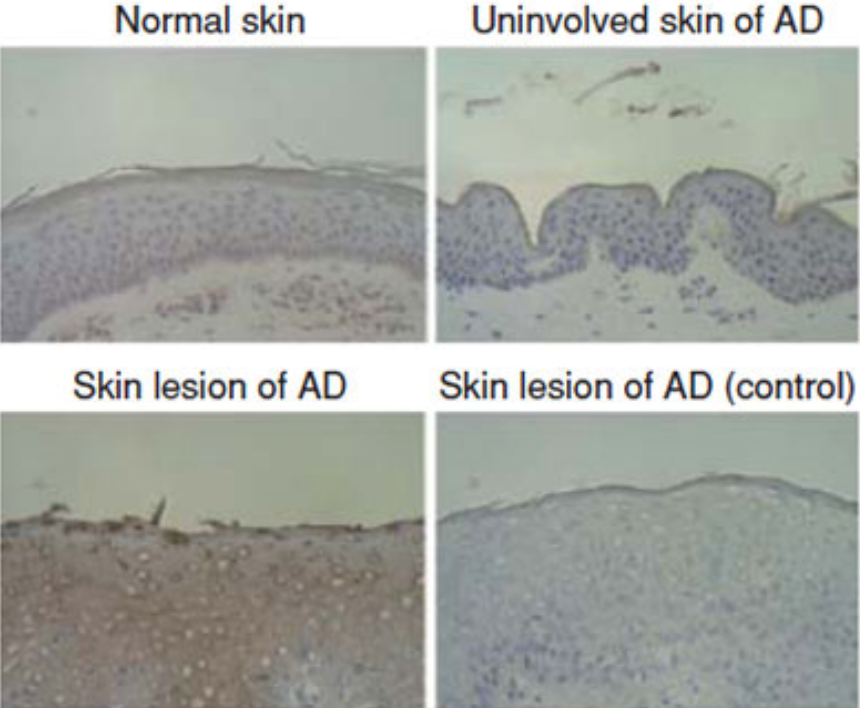 |
| Immunohistochemical staining of squamous cell carcinoma antigen (SCCA) in atopic dermatitis (AD) skin and involved skin of AD patients with or without 200-fold diluted anti-SCCA antibody is depicted. Immunoreacted horseradish peroxidase was developed by diamino benzidine (brown color). | |
| Product name | Anti Squamous Cell Carcinoma Antigen 2 (SERPINB3A/SQN-5) pAb (Rabbit, Antiserum) |
| Cat No | CAC-SU-IZ-P03 |
| Description | Squamous cell carcinoma antigen (SCCA) is a member of the ovalbumin family of serine proteinase inhibitors. The protein was isolated from a metastatic cervical squamous cell carcinoma by Kato and Torigoe (1977). SCCA is detected in the superficial and intermediate layers of normal squamous epithelium, whereas the mRNA is detected in the basal and sub-basal levels. The clinical import of SCCA has been as a circulating tumor marker for squamous cell carcinoma, especially those of the cervix, head and neck, lung, and esophagus. Many clinical studies of cervical squamous cell carcinoma show that the percentage of patients with elevated circulating levels of SCCA increases from approximately 12% at stage 0 to more than 90% at stage IV. Levels fall after tumor resection and rise in approximately 90% of the patients with recurrent disease. Similar trends occur in the other types of squamous cell carcinoma, with a maximum sensitivity of approximately 60% for lung, 50% for esophageal, and 55% for head and neck tumors. The neutral form of SCCA (SCCA1, or SERPINB3) is detected in the cytoplasm of normal and some malignant squamous cells, whereas the acidic form (SCCA2, or SERPINB4) is expressed primarily in malignant cells and is the major form found in the plasma of cancer patients. Thus, the appearance of the acidic fraction of SCCA is correlated with more aggressive tumors (summary by Schneider et al., 1995). Anti-Serpinb3a antibody is a rabbit polyclonal antibody which obtained from the immunization with purified E. coli-derived recombinant mouse Serpinb3a. This antibody can be used for the detection of serpinb3a by immunoblotting and immunostaining. References: Sakata, Y et al., Biochem. Biophys. Res. Commun. 324:1340-1345, 2004 |
| Host | Rabbit |
| Species specificity | MS |
| Figure 1 | 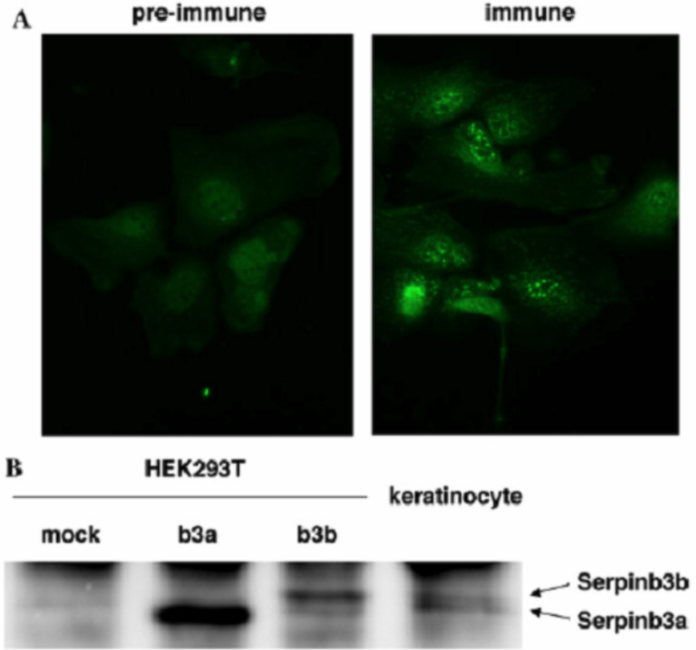 |
| Production and secretion of Serpinb3a and Serpinb3b from mouse keratinocytes. (A) Immunostaining of SCCA-related molecules in mouse keratinocytes by anti-Serpinb3a serum or pre-immune serum is shown. (B) Culture media were prepared from Serpinb3a- or Serpinb3b-transfected HEK293T cells or mouse keratinocytes, and then immunoprecipitates of anti-Serpin3a serum were subjected to Western blotting using anti-Serpinb3a serum. |
|
| Product name | Anti Human Transmembrane Glycoprotein NMB (GPNMB) pAb (Rabbit, Purified Ig) |
| Cat No | CAC-ICA-TG1-RBP1 |
| Description | Melanocyte tumor progression evolves through several distinct stages, from normal cells to highly invasive metastatic melanomas. Markers to help distinguish benign from malignant stages are useful in pathogenetic and diagnostic studies. Weterman et al. (1995) used a subtractive cDNA library to isolate several clones that showed differential expression between high- and low-metastatic melanoma cell lines. They identified 2 genes, which they called NMA and NMB, that showed marked differences between cell lines of varying metastatic capacity. NMB, which was later renamed GPNMB, was preferentially expressed in low-metastatic cell lines. In melanoma metastases there was an inverse relationship between expression of GPNMB compared to calcyclin and thymosin-beta-10, two potential markers for progression of cutaneous melanoma. GPNMB encodes a predicted 560-amino acid transmembrane glycoprotein, the sequence of which is 33% identical to that of the precursor of the melanocyte-specific protein SILV. Northern blot analysis of several human tumors and tumor cell lines failed to detect GPNMB expression with the exception of a histiocytic lymphoma and renal carcinoma cell lines, all derived from the same patient. Two of 3 highly metastatic melanomas expressing recombinant GPNMB showed slower subcutaneous tumor growth, whereas 1 of 3 showed reduced potential for spontaneous metastasis in nude mice. |
| Host | Rabbit |
| Species specificity | HU |
| Figure 1 | 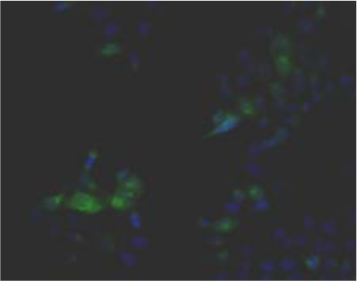 |
| Immunohistochemistry. Hela cells ICAFectin441-transfected with a plasmid encoding human GPNMB were fixed, permeabilized and stained with rabbit anti-human GPNMB polyclonal IgG antibody (diluted 1/100) and Alexa488 goat anti rabbit IgG secondary antibody. Nuclei were counterstained with DAPI. |
|
| Figure 2 | 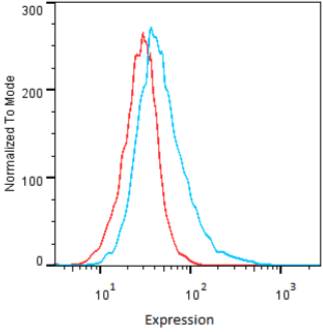 |
| Flow Cytometry. HeLa cells untransfected (red) and ICAFectin441-transfected with a plasmid encoding human GPNMB (blue) were stained with rabbit anti-human GPNMB polyclonal IgG antibody (diluted 1/100) and R-PE goat anti rabbit IgG secondary antibody. |
|
| Figure 3 | 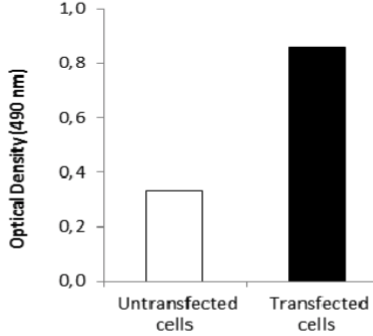 |
| ELISA. Lysates of HeLa cells untransfected (red) and ICAFectin441-transfected with a plasmid encoding human GPNMB were coated in 96 wells plate. Wells were stained with anti-human GPNMB polyclonal IgG and HRP goat anti rabbit IgG secondary antibody. |
|
| Product name | Anti Human/Rat Transmembrane Glycoprotein NMB (GPNMB) pAb (Rabbit, Purified Ig) |
| Cat No | CAC-ICA-TG1-RBP2 |
| Description | Melanocyte tumor progression evolves through several distinct stages, from normal cells to highly invasive metastatic melanomas. Markers to help distinguish benign from malignant stages are useful in pathogenetic and diagnostic studies. Weterman et al. (1995) used a subtractive cDNA library to isolate several clones that showed differential expression between high- and low-metastatic melanoma cell lines. They identified 2 genes, which they called NMA and NMB, that showed marked differences between cell lines of varying metastatic capacity. NMB, which was later renamed GPNMB, was preferentially expressed in low-metastatic cell lines. In melanoma metastases there was an inverse relationship between expression of GPNMB compared to calcyclin and thymosin-beta-10, two potential markers for progression of cutaneous melanoma. GPNMB encodes a predicted 560-amino acid transmembrane glycoprotein, the sequence of which is 33% identical to that of the precursor of the melanocyte-specific protein SILV. Northern blot analysis of several human tumors and tumor cell lines failed to detect GPNMB expression with the exception of a histiocytic lymphoma and renal carcinoma cell lines, all derived from the same patient. Two of 3 highly metastatic melanomas expressing recombinant GPNMB showed slower subcutaneous tumor growth, whereas 1 of 3 showed reduced potential for spontaneous metastasis in nude mice. |
| Host | Rabbit |
| Species specificity | HU RT |
| Figure 1 | 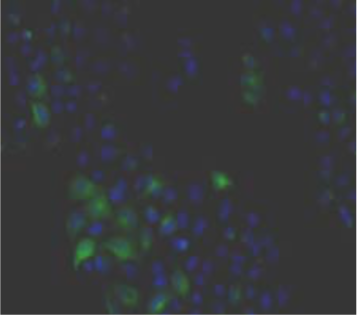 |
| Immunohistochemistry. Hela cells ICAFectin441-transfected with a plasmid encoding human GPNMB were fixed, permeabilized and stained with rabbit anti-human GPNMB polyclonal IgG antibody (diluted 1/100) and Alexa488 goat anti rabbit IgG secondary antibody. Nuclei were counterstained with DAPI. |
|
| Figure 2 | 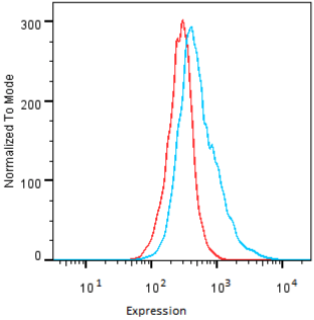 |
| Flow Cytometry. HeLa cells untransfected (red) and ICAFectin441-transfected with a plasmid encoding human GPNMB (blue) were stained with rabbit anti-human GPNMB polyclonal IgG antibody (diluted 1/100) and R-PE goat anti rabbit IgG secondary antibody. |
|
| Figure 3 | 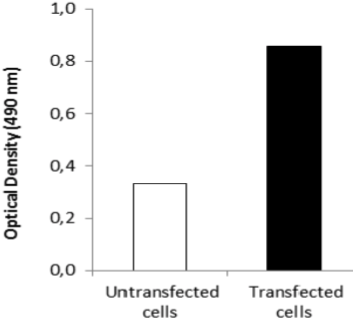 |
| ELISA. Lysates of HeLa cells untransfected (red) and ICAFectin441-transfected with a plasmid encoding human GPNMB were coated in 96 wells plate. Wells were stained with anti-human GPNMB polyclonal IgG and HRP goat anti rabbit IgG secondary antibody. |
|
| Product name | Anti Human/Rat/Mouse Transmembrane Glycoprotein NMB (GPNMB) pAb (Rabbit, Purified Ig) |
| Cat No | CAC-ICA-TG1-RBP3 |
| Description | Melanocyte tumor progression evolves through several distinct stages, from normal cells to highly invasive metastatic melanomas. Markers to help distinguish benign from malignant stages are useful in pathogenetic and diagnostic studies. Weterman et al. (1995) used a subtractive cDNA library to isolate several clones that showed differential expression between high- and low-metastatic melanoma cell lines. They identified 2 genes, which they called NMA and NMB, that showed marked differences between cell lines of varying metastatic capacity. NMB, which was later renamed GPNMB, was preferentially expressed in low-metastatic cell lines. In melanoma metastases there was an inverse relationship between expression of GPNMB compared to calcyclin and thymosin-beta-10, two potential markers for progression of cutaneous melanoma. GPNMB encodes a predicted 560-amino acid transmembrane glycoprotein, the sequence of which is 33% identical to that of the precursor of the melanocyte-specific protein SILV. Northern blot analysis of several human tumors and tumor cell lines failed to detect GPNMB expression with the exception of a histiocytic lymphoma and renal carcinoma cell lines, all derived from the same patient. Two of 3 highly metastatic melanomas expressing recombinant GPNMB showed slower subcutaneous tumor growth, whereas 1 of 3 showed reduced potential for spontaneous metastasis in nude mice. |
| Host | Rabbit |
| Species specificity | HU MS RT |
| Figure 1 | 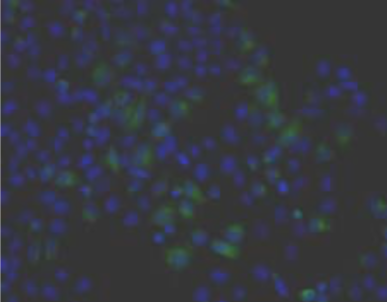 |
| Immunohistochemistry. Hela cells ICAFectin441-transfected with a plasmid encoding human GPNMB were fixed, permeabilized and stained with rabbit anti-human GPNMB polyclonal IgG antibody (diluted 1/100) and Alexa488 goat anti rabbit IgG secondary antibody. Nuclei were counterstained with DAPI. |
|
| Figure 2 | 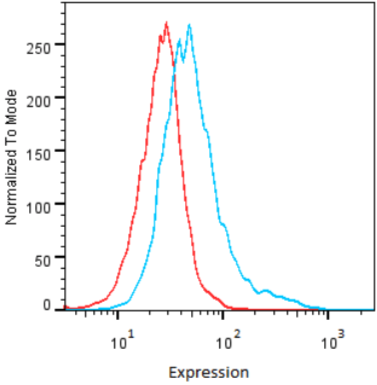 |
| Flow Cytometry. HeLa cells untransfected (red) and ICAFectin441-transfected with a plasmid encoding human GPNMB (blue) were stained with rabbit anti-human GPNMB polyclonal IgG antibody (diluted 1/100) and R-PE goat anti rabbit IgG secondary antibody. |
|
| Figure 3 | 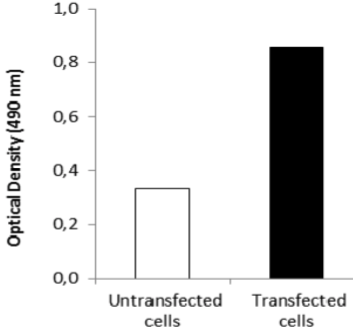 |
| ELISA. Lysates of HeLa cells untransfected (red) and ICAFectin441-transfected with a plasmid encoding human GPNMB were coated in 96 wells plate. Wells were stained with anti-human GPNMB polyclonal IgG and HRP goat anti rabbit IgG secondary antibody. |
|
| Product name | Anti Inter-Alpha-Trypsin Inhibitor Heavy Chain H4 (ITIH4) pAb (Brown Norway Rat, Antiserum) |
| Cat No | CAC-ICA-TG2-RTP1 |
| Description | The inter-alpha-trypsin inhibitors (ITI) are a family of structurally related plasma serine protease inhibitors involved in extracellular matrix stabilization and in prevention of tumor metastasis. The ITI family contains multiple proteins made up of a light chain and a variable number of heavy chains (Salier et al., 1987; Himmelfarb et al., 2004). Peptides derived from the proline-rich potentially active peptide may be biomarkers for a variety of disease states including breast cancer. |
| Host | Rat |
| Species specificity | HU RT |
| Figure 1 | 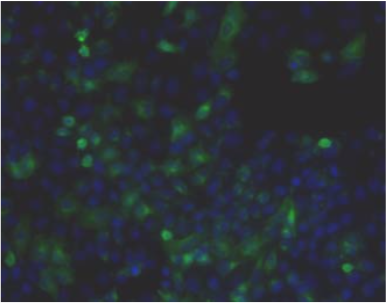 |
| Immunohistochemistry. Hela cells ICAFectin441-transfected with a plasmid encoding human ITIH4 were fixed, permeabilized and stained with rat anti-human ITIH4 serum (diluted 1/50) and Alexa488 goat anti rat IgG secondary antibody. Nuclei were counterstained with DAPI. |
|
| Figure 2 | 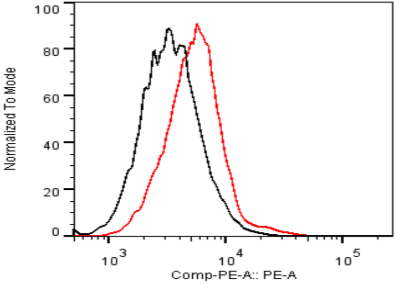 |
| Flow cytometry. Untransfected (black) and transfected with ICAfectin 441 and plasmid encoding human ITIH4 (red) Hela cells were fixed, permeabilized and stained with rat anti-human ITIH4 Polyclonal Antibodies diluted 1/100 and R-PE goat anti rat IgG secondary antibody. |
|
| Figure 3 | 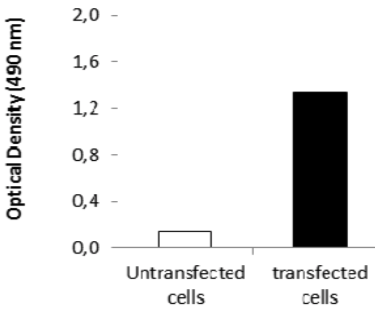 |
| ELISA. Lysates of HeLa cells untransfected (red) and ICAFectin441-transfected with a plasmid encoding human ITIH4 were coated in 96 wells plate. Wells were stained with rat anti-human ITIH4 serum and HRP goat anti rat IgG secondary antibody. |
|
| Product name | Anti Inter-Alpha-Trypsin Inhibitor Heavy Chain H4 (ITIH4) pAb (Sprague Dawley Rat, Antiserum) |
| Cat No | CAC-ICA-TG2-RTP2 |
| Description | The inter-alpha-trypsin inhibitors (ITI) are a family of structurally related plasma serine protease inhibitors involved in extracellular matrix stabilization and in prevention of tumor metastasis. The ITI family contains multiple proteins made up of a light chain and a variable number of heavy chains (Salier et al., 1987; Himmelfarb et al., 2004). Peptides derived from the proline-rich potentially active peptide may be biomarkers for a variety of disease states including breast cancer. |
| Host | Rat |
| Species specificity | HU RT |
| Figure 1 | 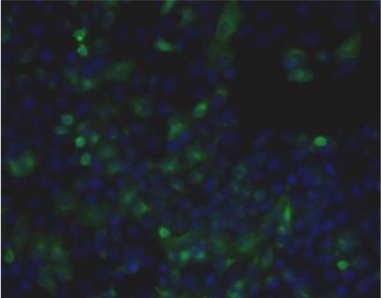 |
| Immunohistochemistry. Hela cells ICAFectin441-transfected with a plasmid encoding human ITIH4 were fixed, permeabilized and stained with rat anti-human ITIH4 serum (diluted 1/50) and Alexa488 goat anti rat IgG secondary antibody. Nuclei were counterstained with DAPI. |
|
| Figure 2 | 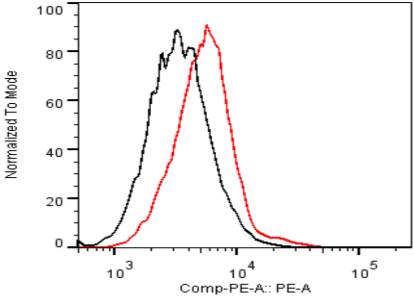 |
| Flow Cytometry. HeLa cells untransfected (black) and ICAFectin441-transfected with a plasmid encoding human ITIH4 (red) were stained with rat anti-human ITIH4 serum (diluted 1/100) and R-PE goat anti rat IgG secondary antibody. |
|
| Figure 3 | 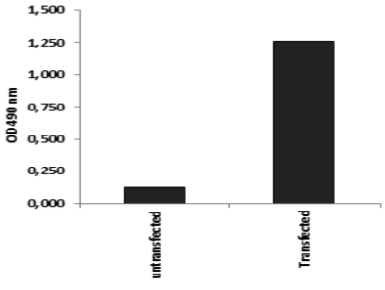 |
| ELISA. Lysates of HeLa cells untransfected (red) and ICAFectin441-transfected with a plasmid encoding human ITIH4 were coated in 96 wells plate. Wells were stained with rat anti-human ITIH4 serum and HRP goat anti rat IgG secondary antibody. |
|
| Product name | Anti Metastasis-Suppressor KiSS-1 (Metastin/Kisspeptin-1) pAb (Rabbit, Antiserum) |
| Cat No | CAC-SK-T01-005 |
| Description | Metastasis suppressor protein in malignant melanomas and in some breast cancers. May regulate events downstream of cell-matrix adhesion, perhaps involving cytoskeletal reorganization. Generates a C-terminally amidated peptide, Metastin, which functions as the endogenous ligand of the G-protein coupled receptor GPR54. Activation of the receptor inhibits cell proliferation and cell migration, key characteristics of tumor metastasis. |
| Host | Rabbit |
| Species specificity | HU |
| Figure 1 | 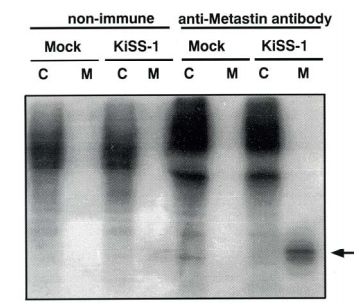 |
| - | |
| Product name | Anti WD Repeat Domain Phosphoinositide-Interacting Protein 3 (WIPI-3/WDR45L) pAb (Mouse, Antiserum) |
| Cat No | CAC-ICA-TG3-MSP1 |
| Description | WD40 repeat proteins are key components of many essential biologic functions. They regulate the assembly of multiprotein complexes by presenting a beta-propeller platform for simultaneous and reversible protein-protein interactions. Members of the WIPI subfamily of WD40 repeat proteins, such as WDR45L, have a 7-bladed propeller structure and contain a conserved motif for interaction with phospholipids (Proikas-Cezanne et al., 2004). By searching a genomic database for sequences similar to WIPI1 followed by RT-PCR of normal testis mRNA, Proikas-Cezanne et al. (2004) cloned WDR45L, which they called WIPI3. The deduced protein contains 7 WD-like repeats. Northern blot analysis detected ubiquitous expression of an approximately 3.0-kb transcript. Highest expression was in heart, skeletal muscle, and pancreas. Proikas-Cezanne et al. (2004) also found that WIPI3 expression was upregulated in a significant portion of ovarian and uterine cancers. |
| Host | Mouse |
| Species specificity | MS |
| Figure 1 | 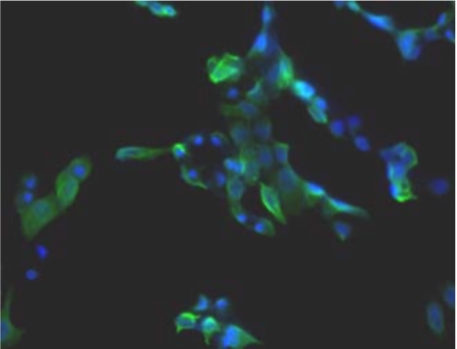 |
| Immunohistochemistry. Hela cells ICAFectin441-transfected with a plasmid encoding mouse WDR45L were fixed, permeabilized and stained with rat anti-human ITIH4 serum (diluted 1/100) and Alexa488 goat anti mouse IgG secondary antibody. Nuclei were counterstained with DAPI. |
|
| Figure 2 | 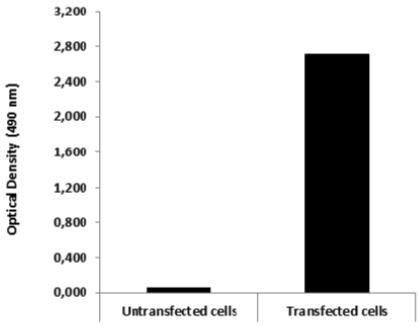 |
| ELISA. Lysates of HeLa cells untransfected and ICAFectin441-transfected with a plasmid encoding mouse WDR45L were coated in 96 wells plate. Wells were stained with mouse anti-mouse WDR45L serum and HRP goat anti rat IgG secondary antibody. |
|
| Product name | Anti Myc Proto-Oncogene Protein (c-Myc) mAb (Clone 9E10) |
| Cat No | CAC-XIM-MA001 |
| Description | c-Myc is a proto-oncogene that encodes a nuclear phosphoprotein that plays a role in cell cycle progression, apoptosis and cellular transformation. The encoded protein forms a heterodimer with the related transcription factor MAX. This complex binds to the E box DNA consensus sequence and regulates the transcription of specific target genes. Amplification of this gene is frequently observed in numerous human cancers. Translocations involving this gene are associated with Burkitt lymphoma and multiple myeloma in human patients. There is evidence to show that translation initiates both from an upstream, in-frame non-AUG (CUG) and a downstream AUG start site, resulting in the production of two isoforms with distinct N-termini. Clone 9E10 recognizes human c-Myc proteins and peptides in random coil rather than alpha-helix structures. References: 1) Mateos-Gomez et al. 2015. Nature. 518(7538):254-7. PMID: 25642960. (WB) 2) Zhang et al. 2014. Genes Dev. 28(8):829-34. PMID: 24736842. (ChIP) 3) Zhou et al. 2012. J Cell Biol. 196(2):203-11. PMID: 22270916. (IP, WB) 4) Mendes et al. 2010. J Biol Chem. 285(50):39117-26. PMID: 20937822. (IP, WB) 5) Royds et al. 1992. J Pathol. 166(3):225-33. PMID: 1381423. (IHC) 6) Spandidos et al. 1987. Anticancer Res. 7(6):1299-304. PMID: 3327455. (IHC) 7) Kari et al. 1986. J Virol. 60(2):345-52. PMID: 3021969. (IP) 8) Evan et al. 1985. Mol Cell Biol. 5(12):3610-6. PMID: 3915782. (WB) |
| Host | Mouse |
| Species specificity | HU |
| Figure 1 | 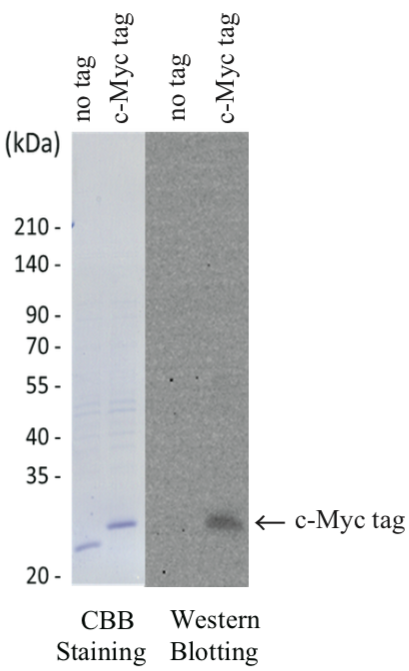 |
| Example Assay Data: 1. Sample Preparation. Inserted c-Myc tag to C-terminus end of DasherGFP plasmid vector (DNA2.0) Protein was expressed in E.coli and lysed (Crude sample). Crude sample as separated by SDS-PAGE. Part of the fraction was used for CBB Staining, rest for Western Blotting. 2. Immunoblotting analysis. Primary Antibody (anti c-Myc [CAC-XIM-MA001]) 1 : 1000 dilution (1μg/mL) Secondary Antibody (anti mouse IgG-HRP, Bethyl Laboratories, Cat.No. A90-216P) 1 : 3000 dilution Anti c-Myc [CAC-XIM-MA001] detection of c-Myc tag: Based on CBB Staining result, the c-Myc tagged fluorescent protein exhibited a higher molecular weight compared to the non-tagged version of fluorescent protein. Further, the Anti c-Myc antibody detected the c-Myc tagged- but not the non-tagged version of fluorescent protein. |
|
| Tumor inhibitors |
|
| Lactoferrin is a member of the transferrin family of genes and its protein product is found in the secondary granules of neutrophils. The protein is a major iron-binding protein in milk and body secretions with an antimicrobial activity, making it an important component of the non-specific immune system. The protein demonstrates a broad spectrum of properties, including regulation of iron homeostasis, host defense against a broad range of microbial infections, anti-inflammatory activity, regulation of cellular growth and differentiation and protection against cancer development and metastasis. Antimicrobial, antiviral, antifungal and antiparasitic activity has been found for this protein and its peptides. Alternatively spliced transcript variants encoding different isoforms have been found for this gene. | |
| Product name | Anti Prohibitin-1 (PHB1) mAb (Clone 1G5C1) |
| Cat No | CAC-CE-051 |
| Description | Prohibitin1 (PHB1), which was initially described as an inhibitor of cell proliferation, is a highly conserved protein found in multiple cellular compartments. In the nucleus it interacts with the transcriptional regulators Rb and E2F1 and controls cell proliferation and apoptosis. References: Schneider et al. (2010) Prohibitin1 acts as a neural crest specifier in Xenopus development by repressing the transcription factor E2F1. Development. 137:4073-4081. |
| Host | RT |
| Species specificity | HU MS RT MKY |
| Figure 1 | 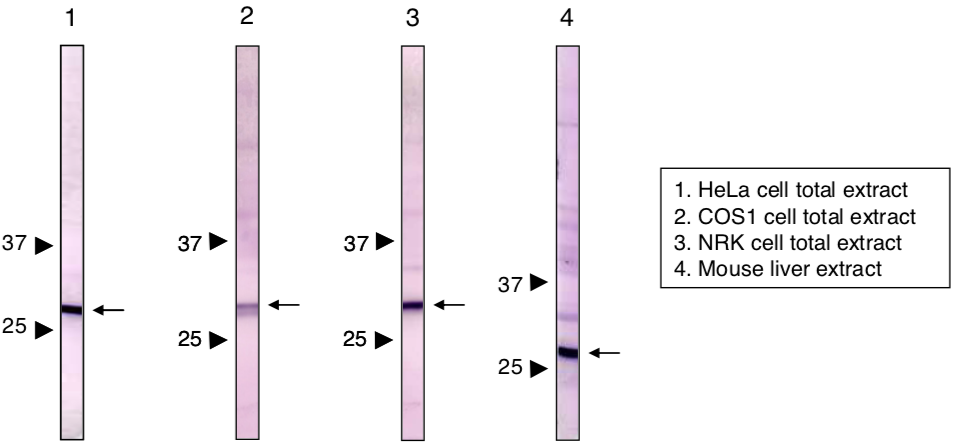 |
| Immunoblot analysis of Prohibitin 1 antibody (1G5C1). 1) HeLa cell extract 2) COS1 cell extract 3) NRK cell extract 4) Mouse liver extract |
|
| Product name | Anti Prohibitin-2 (PHB2) mAb (Clone 7F8B3) |
| Cat No | CAC-CE-052 |
| Description | Prohibitin2 acts as a mediator of transcriptional repression by nuclear hormone receptors via recruitment of histone deacetylases. This protein functions as an estrogen receptor (ER)-selective coregulator that potentiates the inhibitory activities of antiestrogens and represses the activity of estrogens. It is likely involved in regulating mitochondrial respiration and aging. References: Sun et al. (2011) CaMK IV phosphorylates prohibitin 2 and regulates prohibitin 2-mediated repression of MEF2 transcription. Cell Signal. 23:1686-1690. |
| Host | RT |
| Species specificity | HU MS RT MKY |
| Figure 1 | 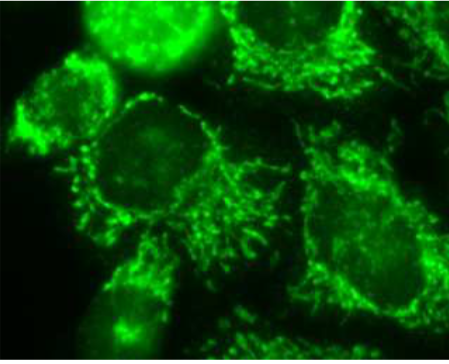 |
| Immunocytochemistry / Immunofluorescence analysis of Prohibitin2 antibody (7F8E3) in HeLa cells. | |
| Figure 2 | 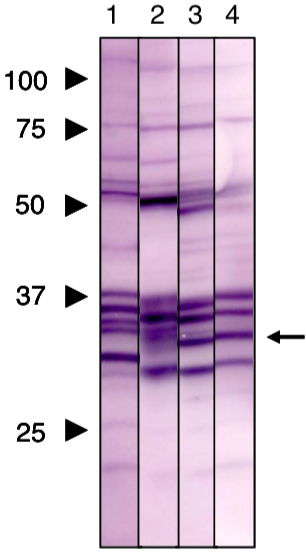 |
| Immunoblot analysis of Prohibitin2 antibody (7F8E3). 1) HeLa cell extract 2) COS1 cell extract 3) NRK cell extract 4) Mouse liver extract |
|
| Tumor promotors |
|
| PACE4 (Proprotein Convertase Subtilisin/Kexin Type 6) is a member of the subtilisin-like proprotein convertase family, which includes proteases that process protein and peptide precursors trafficking through regulated or constitutive branches of the secretory pathway. The protein undergoes an initial autocatalytic processing event in the ER to generate a heterodimer which exits the ER and sorts to the trans-Golgi network where a second autocatalytic event takes place and the catalytic activity is acquired. The protease is constitutively secreted into the extracellular matrix and expressed in many tissues, including neuroendocrine, liver, gut, and brain. This protein is one of the seven basic amino acid-specific members which cleave their substrates at single or paired basic residues. Some of its substrates include transforming growth factor beta related proteins, proalbumin, and von Willebrand factor. PACE4 is thought to play a role in tumor progression and left-right patterning. | |
| Product name | Anti Proprotein Convertase Subtilisin/Kexin Type 6 (PACE4) - HomoB domain pAb (Rabbit, Antiserum) |
| Cat No | CAC-SK-T01-001 |
| Description | PACE4 is a member of the processing protease family that controls cell differentiation and morphogenesis. Its structure and function have been elucidated by Professor Akihiko Tsujij's research group at Tokushima University. PACE4 is believed to play a particularly important role in development and differentiation processes such as organogenesis. In recent years, it has been suggested that it is involved in cancer metastasis and activation of matrix metalloproteinases. Source: Professor Akihiko Tsuji, Faculty of Engineering, Tokushima University. References: 1) Nagahama et al. (1998) Biosynthetic processing and quaternary interaction of proprotein convertase SPC4 (PACE4). FEBS Letters 434, 155-159. 2) Tsuji et al. (2003) Secretory proprotein convertases PACE4 and PC6A are heparin-binding proteins which are localized in the extracellular matrix. Biochim. Biophys. Acta 1645, 95-104. |
| Host | Rabbit |
| Species specificity | HU |
| Product name | Anti Proprotein Convertase Subtilisin/Kexin Type 6 (PACE4) - Propeptide pAb (Rabbit, Antiserum) |
| Cat No | CAC-SK-T01-002 |
| Description | PACE4 is a member of the processing protease family that controls cell differentiation and morphogenesis. Its structure and function have been elucidated by Professor Akihiko Tsujij's research group at Tokushima University. PACE4 is believed to play a particularly important role in development and differentiation processes such as organogenesis. In recent years, it has been suggested that it is involved in cancer metastasis and activation of matrix metalloproteinases. Source: Professor Akihiko Tsuji, Faculty of Engineering, Tokushima University. References: Taniguchi et al (2002) A critical role for the carboxy terminal region of the proprotein convertase, PACE4A, in the regulation of its autocatalytic activation coupled with secretion. Biochem. Biophys. Res. Commun. 290, 878-884. |
| Host | Rabbit |
| Species specificity | HU |
